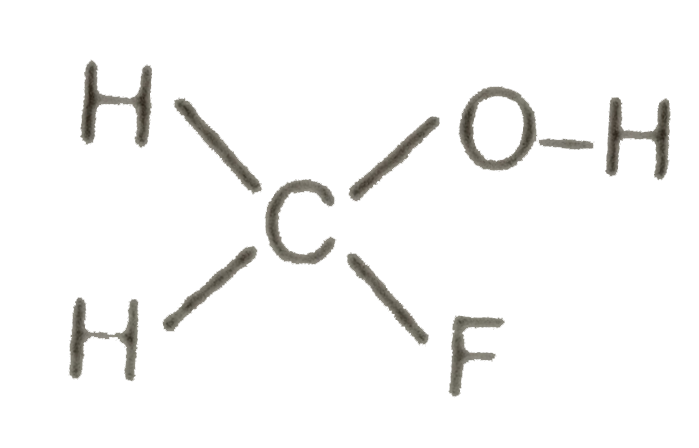
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section- I (Subjective Type Questions)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section - G (Interger Answer Type Questions)|8 VideosBIOMOLECULES
AAKASH INSTITUTE|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section - H (Multiple True-False Type Questions) Identify the correct combination of true(T) and false(F) of the given three statements.
- Statement-1 : Strength of H-bonding depends upon temperature . State...
Text Solution
|
- Statement-1 : In the molecule will form intramolecular H-bonding to...
Text Solution
|
- Statement-1 : All hybrid orbitals of same composition have same shape ...
Text Solution
|
- Statement-1 : Intramolecular H-bonding have no effect on boiling point...
Text Solution
|
- Statement-1 : NH(3) is pyramidal in shape . Statement-2 : SiCl(4) an...
Text Solution
|