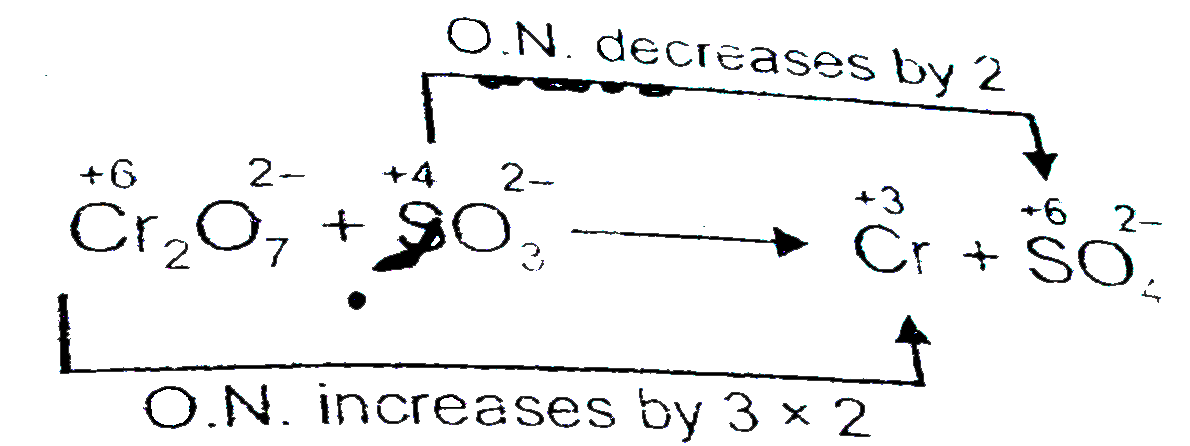Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
AAKASH INSTITUTE|Exercise Try Yourself|28 VideosREDOX REACTIONS
AAKASH INSTITUTE|Exercise Assignment (Section A) (Objective type Questions (one option is correct))|40 VideosPRINCIPLES OF QUALITATIVE ANALYSIS
AAKASH INSTITUTE|Exercise Assignment (SECTION H)|9 VideosSOLUTIONS
AAKASH INSTITUTE|Exercise ASSIGMENT (SECTION-J) AAKASH CHALLENGERS QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-REDOX REACTIONS-ASSIGNMENT SECTION - D
- Balance the net equtation fro th reaction of potassium dichromate (VI)...
Text Solution
|
- A : Fluorine acts as a stronger oxidising agent than chlorine. R : S...
Text Solution
|
- A : Oxidation number of carbon in HCN is +4. R : Oxidation state and...
Text Solution
|
- A : Equivalent weight of KMnO(4) in acidic medium is M/5. R : In aci...
Text Solution
|
- A : Electrons flow in external circuit of galvanoic cell while ions fl...
Text Solution
|
- A : Sn^(2+)" and "Fe^(3+) can't remain together in a solution. R : S...
Text Solution
|
- A : The oxidation number of S is +6 in H(2)SO(4). R : H(2)SO(4) has ...
Text Solution
|
- A : HNO(2) acts as reducing agent only. R : HNO(2) oxidises to HNO(3...
Text Solution
|
- A : In alkaline medium, KMnO(4) acts as powerful oxidising agent. R ...
Text Solution
|
- A : When Cu(2)S is converted into Cu^(++)" & " SO(2), then equivalent ...
Text Solution
|
- A : I(2) is a mild oxidising agent. R : I(2) can be used for titrati...
Text Solution
|