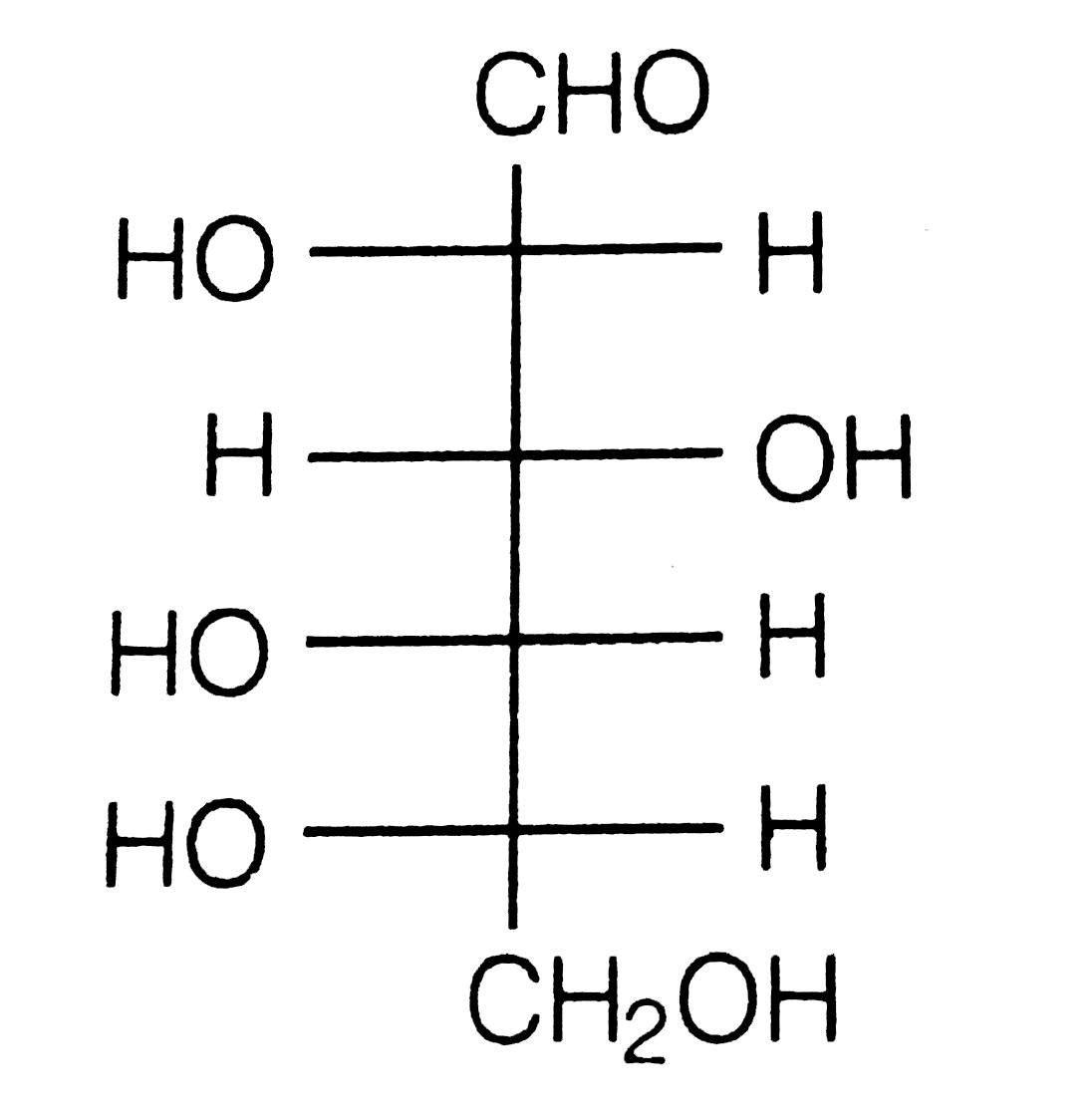
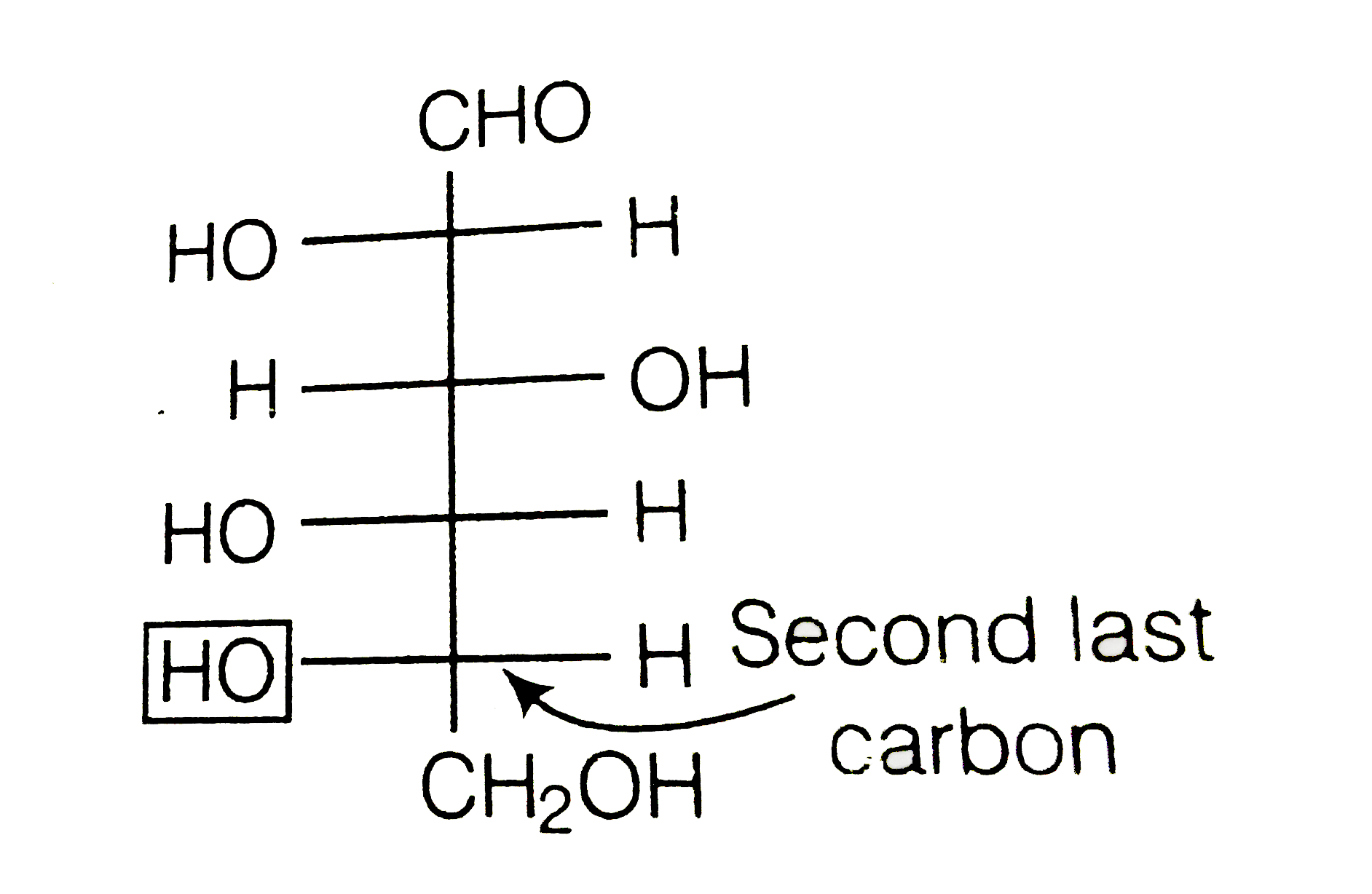Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
NCERT EXEMPLAR ENGLISH|Exercise Matching The Columns|2 VideosBIOMOLECULES
NCERT EXEMPLAR ENGLISH|Exercise Assertion and Reason|7 VideosBIOMOLECULES
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|5 VideosAMINES
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER|3 VideosCHEMICAL KINETICS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-BIOMOLECULES -Short Answer Type Questions
- Under what condtions glucose is converted to gluconic acid and sacchar...
Text Solution
|
- Monosaccharides contain carbonyl group hence are classified, as aldose...
Text Solution
|
- The letters 'D' or 'L' before the name of a stereoisomer of a compound...
Text Solution
|
- Aldopentoses named as ribose and 2-deoxyribose are found in nucleic ac...
Text Solution
|
- Which sugar is called invert sugar? Why is it called so?
Text Solution
|
- Amino acids can be classified as alpha-,beta-,gamma-,delta-and so depe...
Text Solution
|
- alpha-helix is a secondary structure of proteins formed by twisting of...
Text Solution
|
- Some enzymes are named after the reaction, where they are used. What n...
Text Solution
|
- During curdling of milk, what happens to sugar present in it?
Text Solution
|
- How do yout explain the presence of five -OH groups in glucose molecul...
Text Solution
|
- Why does compound (A) given below not forman oxime?
Text Solution
|
- Why must vitamin C be supplied regularly in diet?
Text Solution
|
- Sucrose is dextrorotatory but the mixture obtained after hydrolysis is...
Text Solution
|
- Amino acids behave like salts rather than simple amines or carboxylic ...
Text Solution
|
- Structure of glycine and alanine are given below. Show the peptide lin...
Text Solution
|
- Protein found in a biological system with a unique three-dimensional s...
Text Solution
|
- Activation energy for the acid catalysed hydrolysis of sucrose is 6.22...
Text Solution
|
- How do you explain the presence of an aldehydic group in a glucose mol...
Text Solution
|
- Which moieties of nucleosides are involved in the formation of phospho...
Text Solution
|
- What are glucosidic linkages? In which type of biomolecules are they p...
Text Solution
|