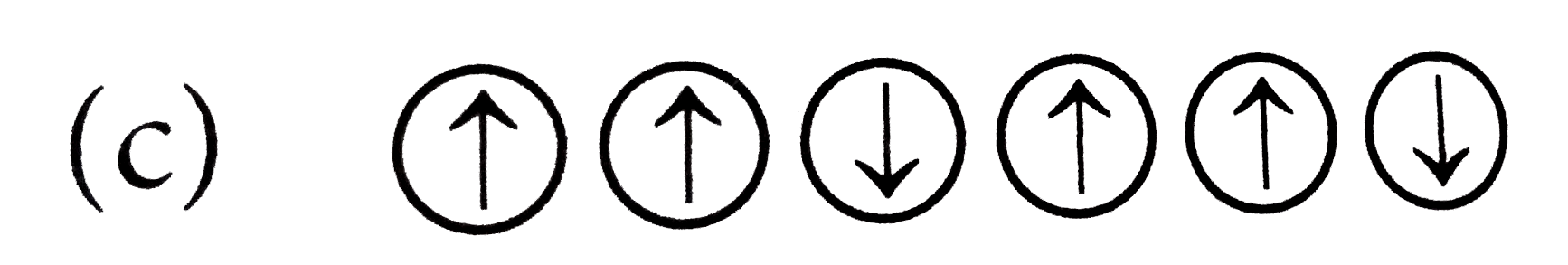
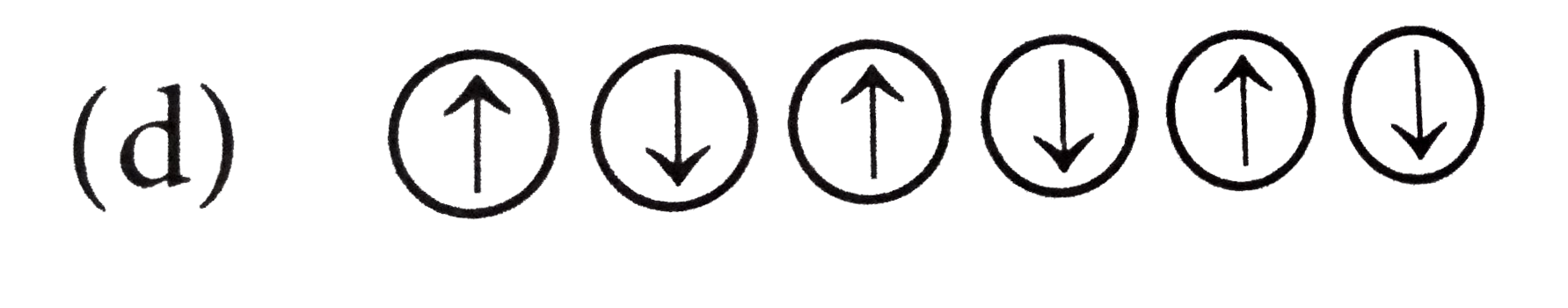
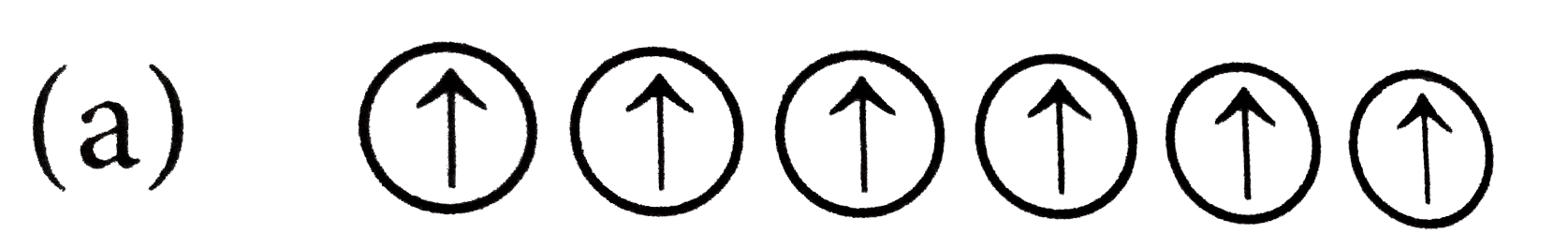A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise Amorphous And Crystalline Solids|3 VideosTHE SOLID STATE
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE SOLID STATE -EXEMPLAR PROBLEMS
- which of the following is not a characteristic of a crystalline s...
Text Solution
|
- Which of the following is an amorphous solid
Text Solution
|
- Which of the following arrangements shows schematic alignment of magne...
Text Solution
|
- which of the following is true about the value of refractive in...
Text Solution
|
- Which of the following statement is not true about amorphous solids?
Text Solution
|
- The sharp melting point of crystalline solids compared to amorphous so...
Text Solution
|
- Iodine molecules are held in the crystal lattice by:
Text Solution
|
- which of the following is a network solid?
Text Solution
|
- which of the following solids is not an electrical conductor ? ...
Text Solution
|
- which of the following is not the characteristic of ionic solids?
Text Solution
|
- Graphite is a good conductor of electricity due to the presence of :
Text Solution
|
- which of the following oxides behaves as conductor or insulator ...
Text Solution
|
- Which of the following oxides shows electrical properties like metals ...
Text Solution
|
- The lattice site in a pure crystal cannot be occupied by :
Text Solution
|
- Graphite cannot be classified as :
Text Solution
|
- Cations are present in the interstitial sites in …………… .
Text Solution
|
- Schottky defect is observed in crystals when ……………. .
Text Solution
|
- which of the following is true about the charge acquired by p- t...
Text Solution
|
- To get a n- type semiconductor from silicon , it should be doped...
Text Solution
|
- The total of tetrahedral voids in the face centred unit cell is ……...
Text Solution
|