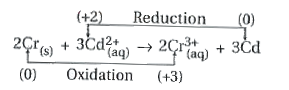Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (MCQ.s)|17 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (MCQ.s More Than One Options)|10 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-B (Intext Questions and Answers)|14 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-C Textual Exercise
- Arrange the following metals in the order in which they displace each ...
Text Solution
|
- Given the standard electrode potentials: K^(+)|K=-2.93V, Ag^(+)|Ag...
Text Solution
|
- Depict the galvanic cell in which the reaction Zn((S))+Ag((aq))^(+)to ...
Text Solution
|
- Calculate the standard cell potentials of galvanic cells in which the ...
Text Solution
|
- Write the Nernst equation and emf of the following cells at 298 K: (...
Text Solution
|
- In the button cells widely used in watches and other devices the follo...
Text Solution
|
- The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm^(-1...
Text Solution
|
- The resistance of a conductivity cell containing 0.001 M KCl solution ...
Text Solution
|
- The conductivity of sodium chloride at 298K has been determined at dif...
Text Solution
|
- Conductivity of 0.00241 M acetic acid is 7.896xx10^(-5)" S "cm^(-1). C...
Text Solution
|
- How much charge is required for the following reductions: (i) 1 mol ...
Text Solution
|
- How much electricity in terms of Faraday is required to produce: (i)...
Text Solution
|
- How much electricity is required in coulomb for the oxidation of (i)...
Text Solution
|
- A solution of Ni(NO(3))(2) is electrolysed between platinum electrodes...
Text Solution
|