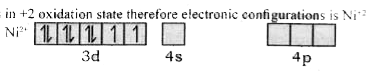Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)
SUNSTAR PUBLICATION|Exercise PART - D|22 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)
SUNSTAR PUBLICATION|Exercise PART - B|8 VideosII PUC CHEMISTRY ( ANNUAL EXAM QUESTION PAPER MARCH - 2020)
SUNSTAR PUBLICATION|Exercise PART-D|32 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)
SUNSTAR PUBLICATION|Exercise PART - D|26 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)-PART - C
- Draw labelled diagram of Hall-Heroult electrolytic cell for the extrac...
Text Solution
|
- For the manufacture of Ammonia by Haber's process, write the equation ...
Text Solution
|
- Write the equation for i) The action of SO(2) with chlorine in the p...
Text Solution
|
- Complete the following equation : i) underset("(Cold and dilute)")(2...
Text Solution
|
- Explain the manufacture of Potassium dichromate from chromite ore.
Text Solution
|
- With reference to the first row transition series: i) Name the metal...
Text Solution
|
- Using valence bond theory account for the geometry and magnetic nature...
Text Solution
|
- Give the IUPAC name of [Ti(H(2)O)(6)]^(3+). Draw cis and trans isomers...
Text Solution
|