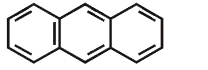
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
JEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise SECTION-B|50 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION-A)|20 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise Chemistry (Section B)|10 VideosJEE MAIN
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY|150 VideosJEE MAIN 2022
JEE MAINS PREVIOUS YEAR|Exercise Question|561 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2021-SECTION-A
- Match List-I and List-II.
Text Solution
|
- Match List-I with List-II. Choose the correct answer from the opt...
Text Solution
|
- Which one of the following compounds is nonaromatic ?
Text Solution
|
- What is the correct order of the following elements with respect to th...
Text Solution
|
- Given below are two statements : Statement I : The value of the para...
Text Solution
|
- The incorrect statement among the following is :
Text Solution
|
- The correct shape and I-I-I bond angles respectively in I(3)^(-) ion a...
Text Solution
|
- Given below are two statements : one is labelled as Assertion A and th...
Text Solution
|
- The diazonium salt of which of the following compound will form a colo...
Text Solution
|
- The correct set from the following in which both pairs are in correct ...
Text Solution
|
- Which among the following species has unequal bond lengths ?
Text Solution
|
- Carbylamine test is used to detect the presence of primary amino group...
Text Solution
|
- Which of the following is correct structure of alpha-anomer of maltose...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- The correct sequence of reagents used in the preparation of 4-bromo-2-...
Text Solution
|
- Water does not produce CO on reacting with
Text Solution
|
- The correct order of acid character of the following compounds is ...
Text Solution
|
- Correct statement about the given chemical reaction is :
Text Solution
|
- Correct order of bond dissociation energy of Halogen
Text Solution
|
- Given below are two statements : Statement I : The pH of rain wate...
Text Solution
|