Topper's Solved these Questions
JEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY SECTION A|60 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY SECTION B|30 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION A)|40 VideosJEE MAIN
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY|150 VideosJEE MAIN 2022
JEE MAINS PREVIOUS YEAR|Exercise Question|561 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2021-CHEMISTRY (SECTION B)
- Two salts A(2)X and MX have the same value of solubility product of 4....
Text Solution
|
- 2Mn O (4) ^(-) + b C(2) O (4) ^(2-) + cH^(+) to x Mn ^(2+) + y CO (2) ...
Text Solution
|
- AB(2) is 10% dissociated in water to A^(2+) and B^(-) The boiling po...
Text Solution
|
- The equivalents of ethylene diamine required to replace the neutral li...
Text Solution
|
- The decomposition of formic acid on gold surface follows first order k...
Text Solution
|
- When light of wavelength 248 nm falls on a metal of threshold energy 3...
Text Solution
|
- Complete combustion of 750 g of an organic compound provides 420 g of ...
Text Solution
|
- A 650 molar solution of KOH (aq.) has a density of1.89 g cm ^(-3). The...
Text Solution
|
- For the reaction A(g) = B (g) at 495 K, Delta (r ) G ^(@)=- 9. 478 kJ ...
Text Solution
|
- A certain element crystallises in a bcc lattice of unit cell edge leng...
Text Solution
|
- A certain orbital has n = 4 and m(L) = -3 . The number of radial nodes...
Text Solution
|
- The pressure exerted by a non-reactive gaseous mixture of 6.4g of meth...
Text Solution
|
- The oxygen dissolved in water exerts a partial pressure of 20 kPa in t...
Text Solution
|
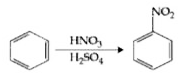
- In the above reaction, 3.9 g of benzene on nitration gives 4.92 g of n...
Text Solution
|
- For a certain first order reaction 32% of the reactant is left after ...
Text Solution
|
- 15 mL of aqueous solution of Fe^(2+) in acidic medium completely react...
Text Solution
|
- 0.01 moles of a weak acid HA ( K(a) = 2.0 xx 10^(-6) ) is dissolved in...
Text Solution
|
- The mole fraction of a solution in a 100 molal aqueous solution is ""x...
Text Solution
|
- The standard enthalpies of formation of Al(2) O(3) and CaO are - 1675 ...
Text Solution
|
- The reaction of white phosphorus on boiling with alkali in inert atmos...
Text Solution
|