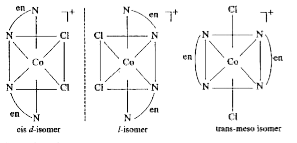Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER 2015
ICSE|Exercise PART-II SECTION-B QUESTION 6. |4 VideosSAMPLE PAPER 2015
ICSE|Exercise PART-II SECTION-B QUESTION 7. |3 VideosSAMPLE PAPER 2015
ICSE|Exercise PART-II SECTION-A QUESTION 4. |7 VideosSAMPLE PAPER 2014
ICSE|Exercise PART-II (SECTION -C)|21 VideosSAMPLE PAPER 2016
ICSE|Exercise PART-II SECTION-C QUESTION 10. |7 Videos
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2015-PART-II SECTION-B QUESTION 5. (ANSWR ANY TWO QUESTIONS)
- Write the IUPAC names of the following coordination compound [Cr(NH3)...
Text Solution
|
- Write the IUPAC names of the following coordination compounds : [Pt...
Text Solution
|
- State the hybridization and magnetic property of [Fe(CN)6]^(3-) ion ac...
Text Solution
|
- What type of structural isouters are [Co(NH3)5Br]SO4 and Co[(NH3)5SO4]...
Text Solution
|
- Draw all the isomers (geometrical and optical) of: (i) [CoCl(2)(en)(...
Text Solution
|