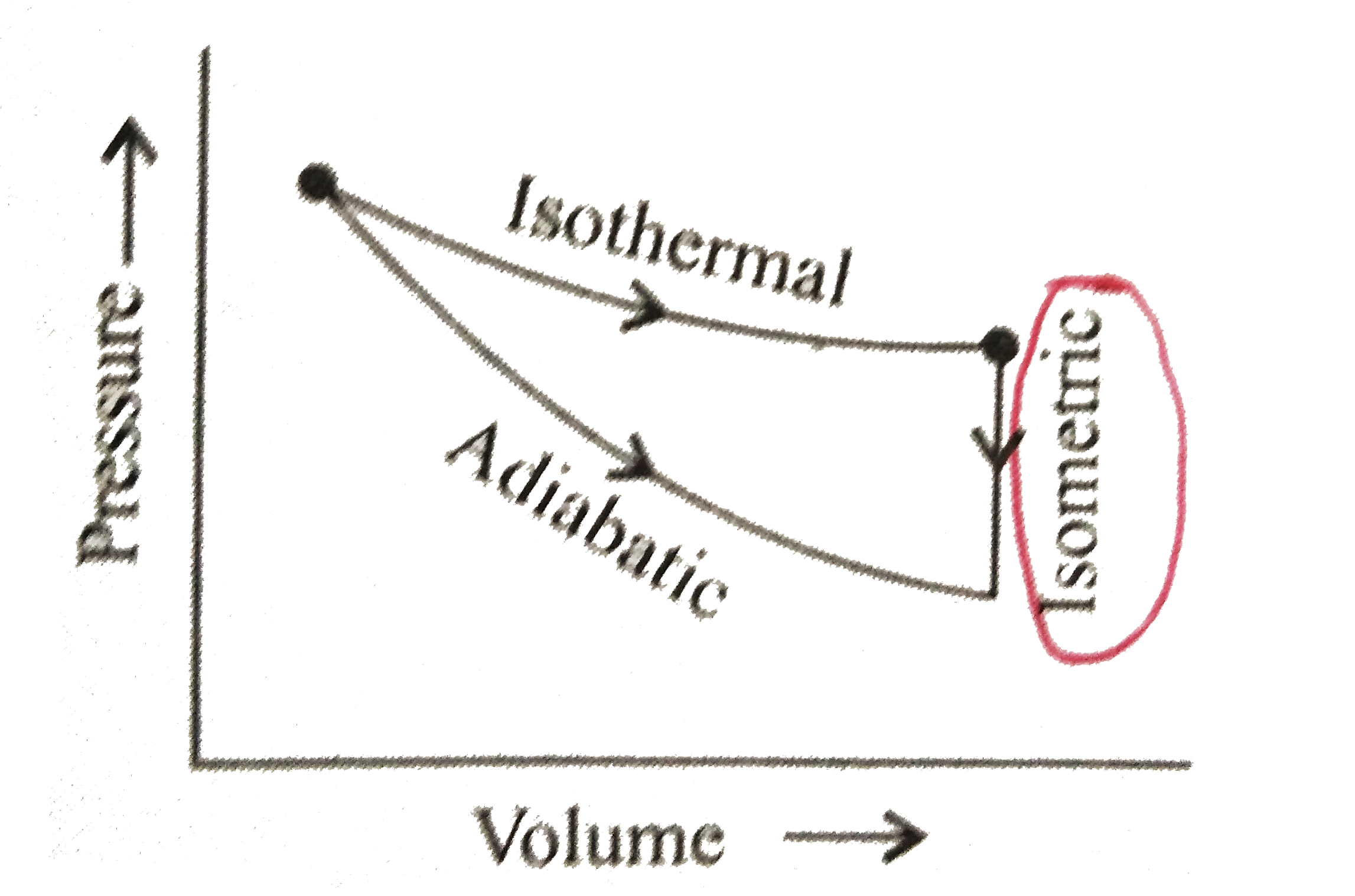
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-THERMODYNAMICS-Ex 6.1
- 500cm^(3) of a sample of an ideal gas is compressed by an average pres...
Text Solution
|
- Three moles of an ideal gas are expanded isothermally and reversibly a...
Text Solution
|
- 2.8g of N(2) gas at 300K and 20atm was allowed to expand isothermally ...
Text Solution
|
- State whther each of the following will increase or decreases the tota...
Text Solution
|
- Two moles of an idela gas at 2atm and 27^(@)C is compressed isothermal...
Text Solution
|
- A simple of gas present in a cylinder fitted with a frictionless pisto...
Text Solution
|
- One mole of an ideal mono-atomic gas at 27^(@)C expands adiabatically ...
Text Solution
|
- A 5L cylinder contained 10 moles of oxygen gas at 27^(@)C. Due to sudd...
Text Solution
|
- Adiabatic expansion of an ideal gas is accompanied by
Text Solution
|
- For a cyclic process, which of the following is true?
Text Solution
|
- One mole of a gas is heated at constant pressure to raise its temperat...
Text Solution
|
- What work is to be done on 2mol of a perfect gas at 27^(@)C it is comp...
Text Solution
|
- 1mol of an ideal gas undergoes reversible isothermal expansion form an...
Text Solution
|
- A sample of 2kg of helium (assumed ideal) is taken through the process...
Text Solution
|
- An ideal diatomic gas is caused to pass throguh a cycle shown on the P...
Text Solution
|
- A heat engine carries one mole of an ideal mono-atomic gas around the ...
Text Solution
|
- One mole of an ideal mono-atomic gas is caused to go through the cycle...
Text Solution
|
- Calculate the work done by 1.0 mol of an ideal gas when it expands at ...
Text Solution
|
- 3.0 moles of an ideal gas at 27^(@)C is compressed at constant temp. r...
Text Solution
|
- When 3mole of an idela gas expand reversibly and isothermally five tim...
Text Solution
|