A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct(Chemical Bonding )|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct (Dipole Moment)|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2 .2 Objective|34 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Linked Comprehension
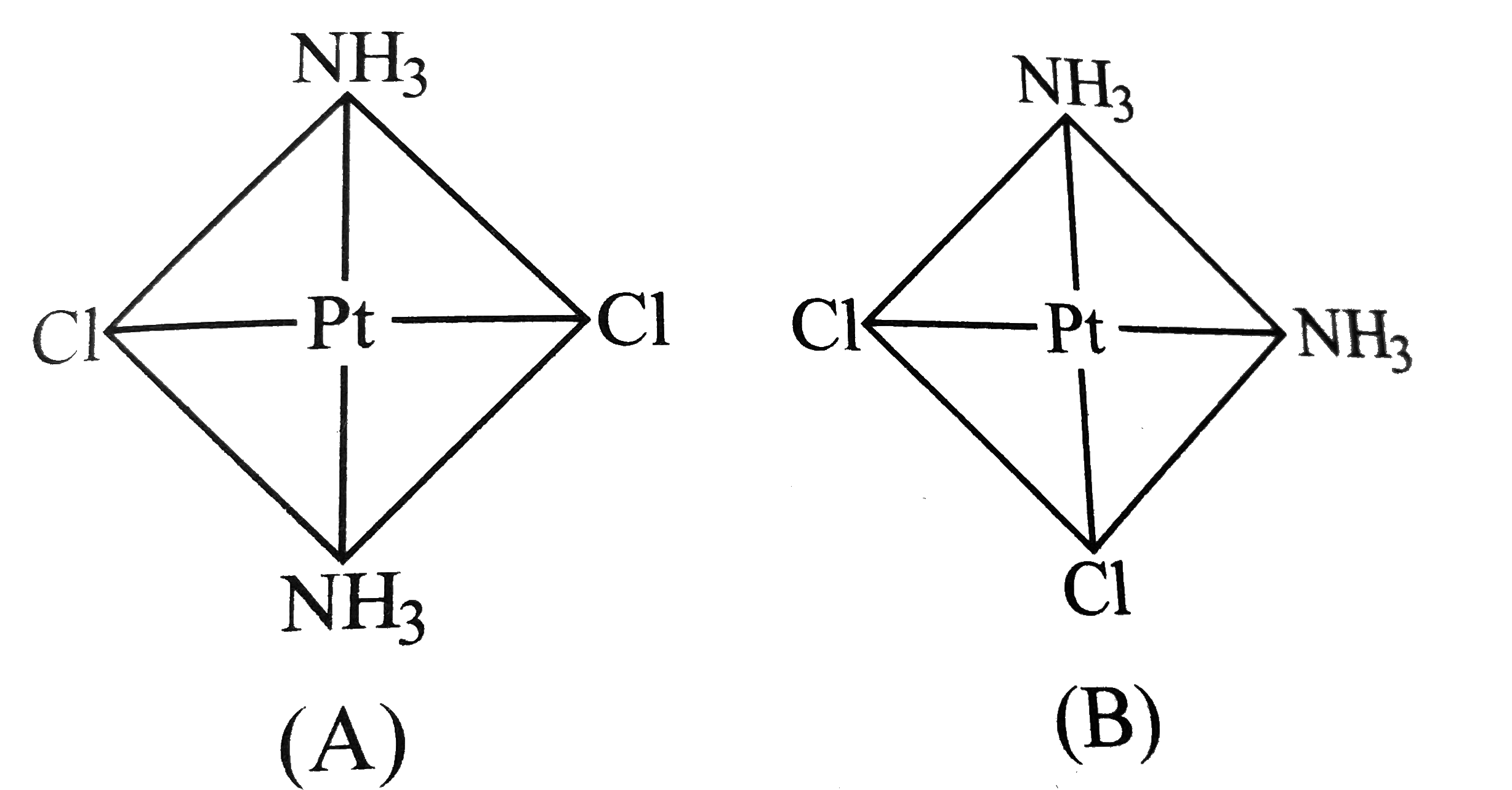
- The Pt-CI distance is 2.32 A in several crystaline compounds What is...
Text Solution
|
- The platinum-chlorine distance has been found to be 2.32Å in several...
Text Solution
|
- The platinum-chlorine distance has been found to be 2.32Å in several...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and liquid HF but not in the dilute aque...
Text Solution
|
- Consider the following molecules . A:Anti -pyridine -2-carboxaldoxime...
Text Solution
|
- Consider the following molecules . A:Anti -pyridine -2-carboxaldoxime...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Which of the following statements is correct about O(2),O(2)^(Θ),O(2)^...
Text Solution
|
- O(2)^(2-) will have .
Text Solution
|
- According to the moleular orbital theory, all atomic orbitals combine ...
Text Solution
|
- According to the moleular orbital theory, all atomic orbitals combine ...
Text Solution
|
- Most of the polyatomic molecules except a few such as CO(2) and CS(2) ...
Text Solution
|
- Which of the following have highest bond angle ? .
Text Solution
|
- Which of the following hybridisation may have more than one type of bo...
Text Solution
|

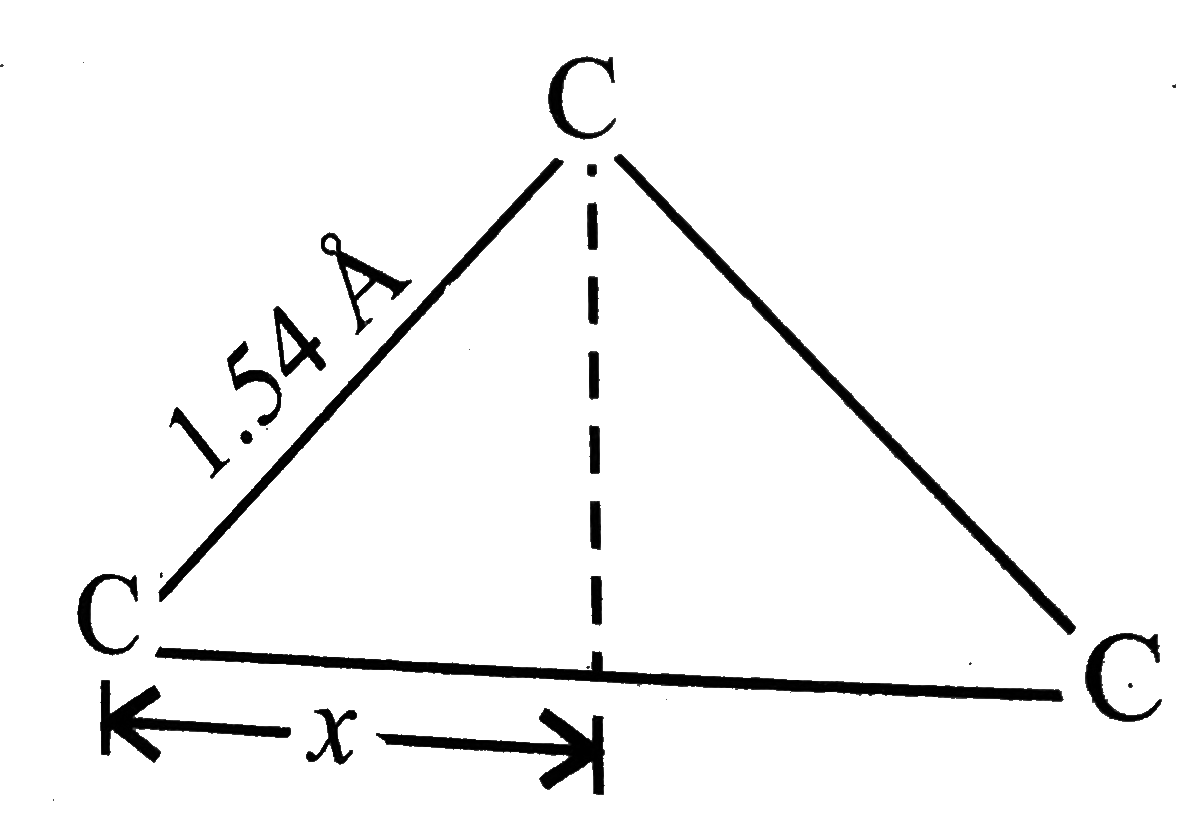 .
.