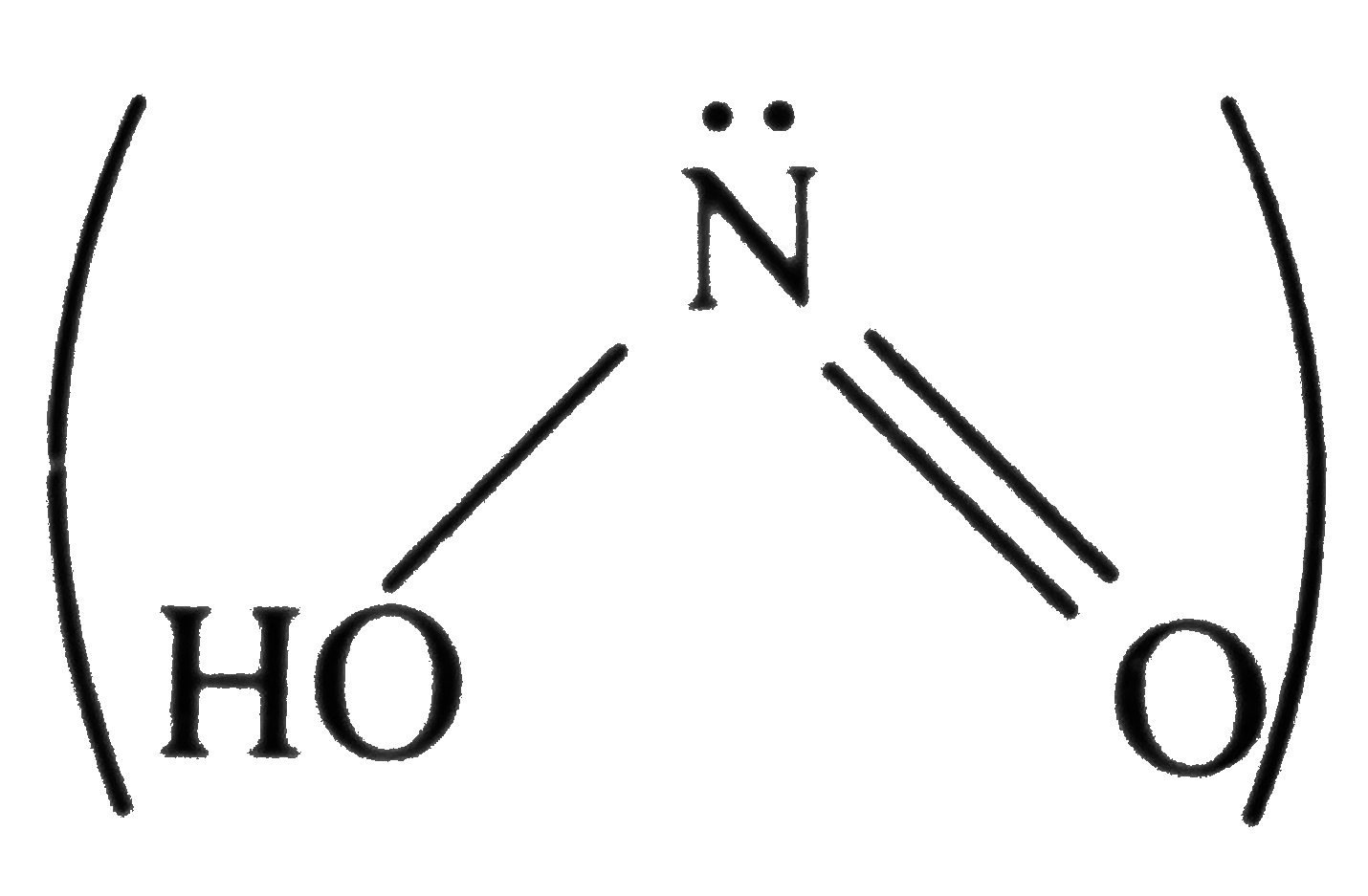
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Dipole Moment)|16 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Hybridisation)|33 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct (Miscellaneous)|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Chemical Bonding)
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- The bond angle between two hybrid orbitals is 180^(@) The percentage ...
Text Solution
|
- Which type of bond is not present in HNO(2) molecule ? .
Text Solution
|
- KF combines with HF to form KHF(2) The compound contains the species .
Text Solution
|
- There is no S-S bond in .
Text Solution
|
- Angle between two hybridised orbital is 105^(@) and hence the percenta...
Text Solution
|
- The octet rule is not valid for the molecule
Text Solution
|
- The total number of electrons that take part in forming the bond in N(...
Text Solution
|
- Bonds presents in CuSO(4) .5H(2)O is
Text Solution
|
- The bond between two identical non-metal atoms has a pair of electrons...
Text Solution
|
- The number and type of bonds between two C-atom in SrC(2) are .
Text Solution
|
- Which species has the maximum number of lone pair of electrons on th...
Text Solution
|
- Among the following the electron deficient compound is
Text Solution
|
- Which of the following does not follow the octet rule ? .
Text Solution
|
- Which of the following does not have coordinate bonds ? .
Text Solution
|
- Which of the following bonds is the strongest ? .
Text Solution
|
- When two atoms combine to form a molecule
Text Solution
|
- Most favourable conditions for inoic bonding are .
Text Solution
|
- Which of the following is not a correct statement ? .
Text Solution
|
- Element A has 3 electrons in the outermost orbit and element B has 6 e...
Text Solution
|