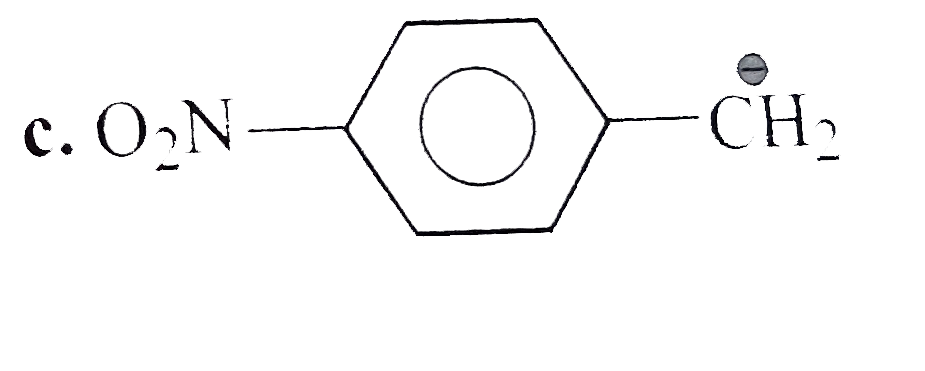
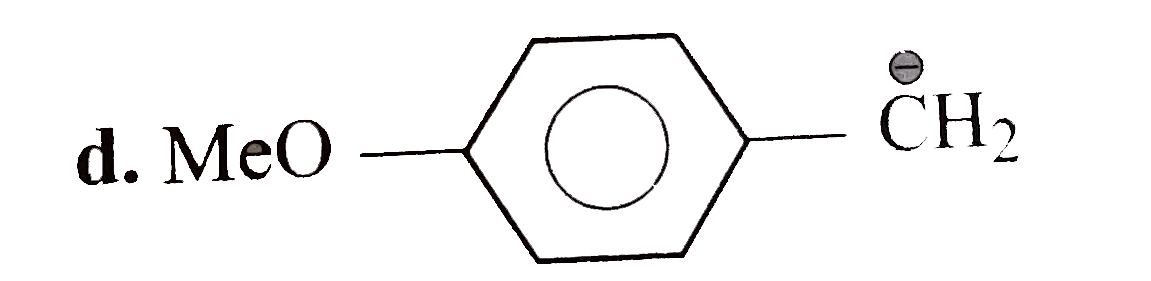
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct|22 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct|23 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Concept|17 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL ORGANIC CHEMISTRY-Comprehension
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- An organic reaction occurs by using reagents called electrophiles and ...
Text Solution
|
- Carbene intermediates are produced by the photolysis of diazomethane (...
Text Solution
|
- Carbene intermediates are produced by the photolysis of diazomethane (...
Text Solution
|
- Carbene intermediates are produced by the photolysis of diazomethane (...
Text Solution
|
- Carbene intermediates are produced by the photolysis of diazomethane (...
Text Solution
|
- Carbene intermediates are produced by the photolysis of diazomethane (...
Text Solution
|
- Consider the following reaction : , . The compound D is an ortho...
Text Solution
|
- Consider the following reaction : , . The m-isomer of D and E i...
Text Solution
|
- Consider the following reaction : , . The decreasing order of a...
Text Solution
|
- Consider the following reaction : , . Which of the above three ...
Text Solution
|
- Consider the Hofmann ammonolysis reaction : R-X +ddotNH(3)rarr under...
Text Solution
|
- Consider the Hofmann ammonolysis reaction : R-X +ddotNH(3)rarr under...
Text Solution
|
- Consider the Hofmann ammonolysis reaction : R-X +ddotNH(3)rarr under...
Text Solution
|
- Consider the Hofmann ammonolysis reaction : R-X +ddotNH(3)rarr under...
Text Solution
|
- Consider the Hofmann ammonolysis reaction : R-X +ddotNH(3)rarr under...
Text Solution
|