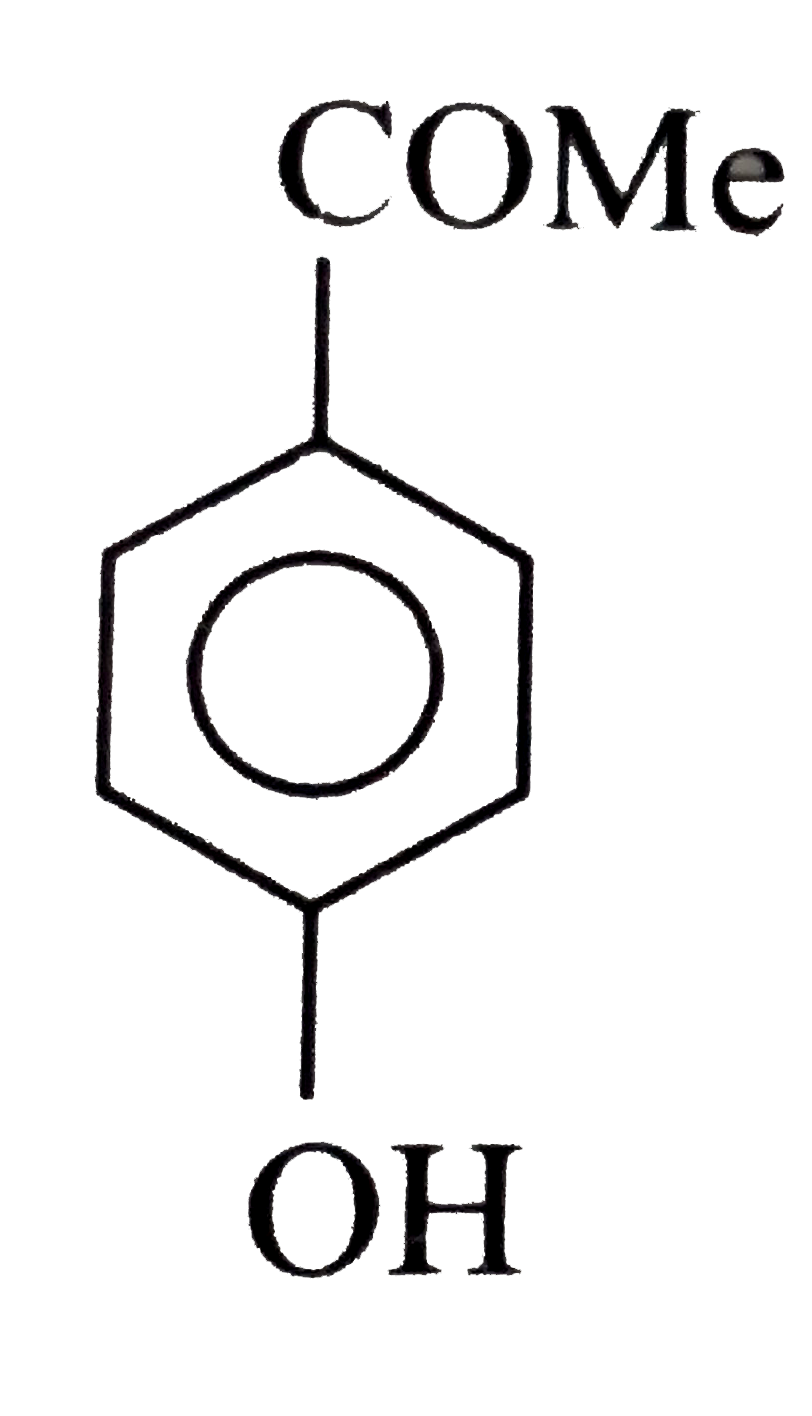
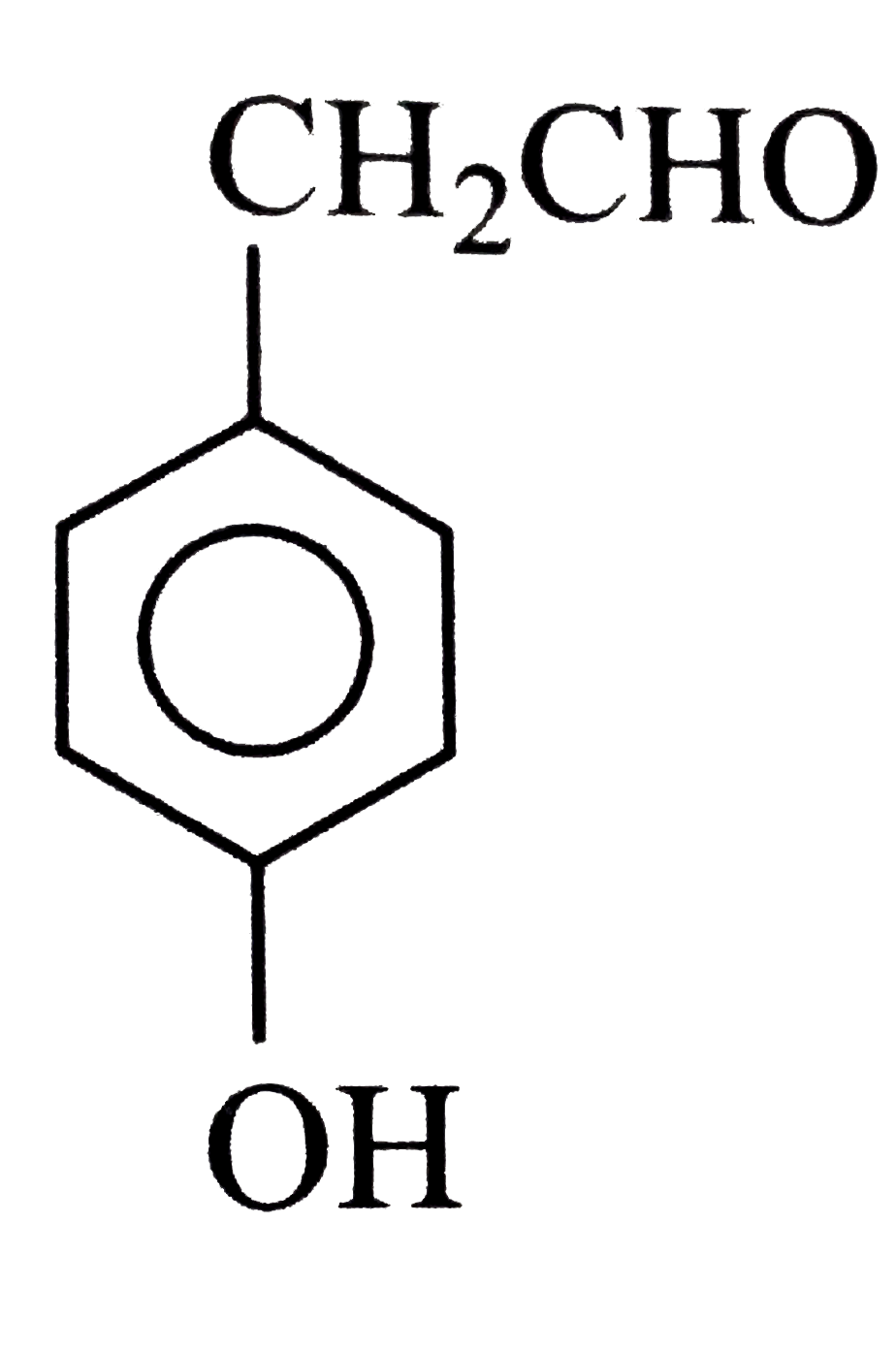
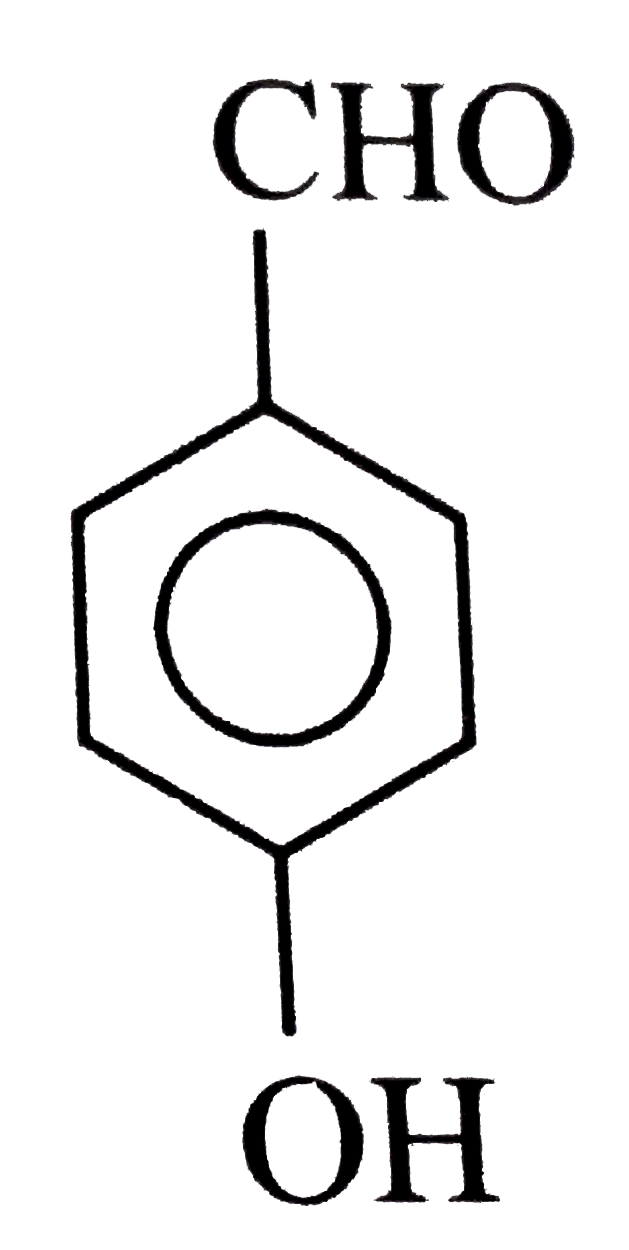
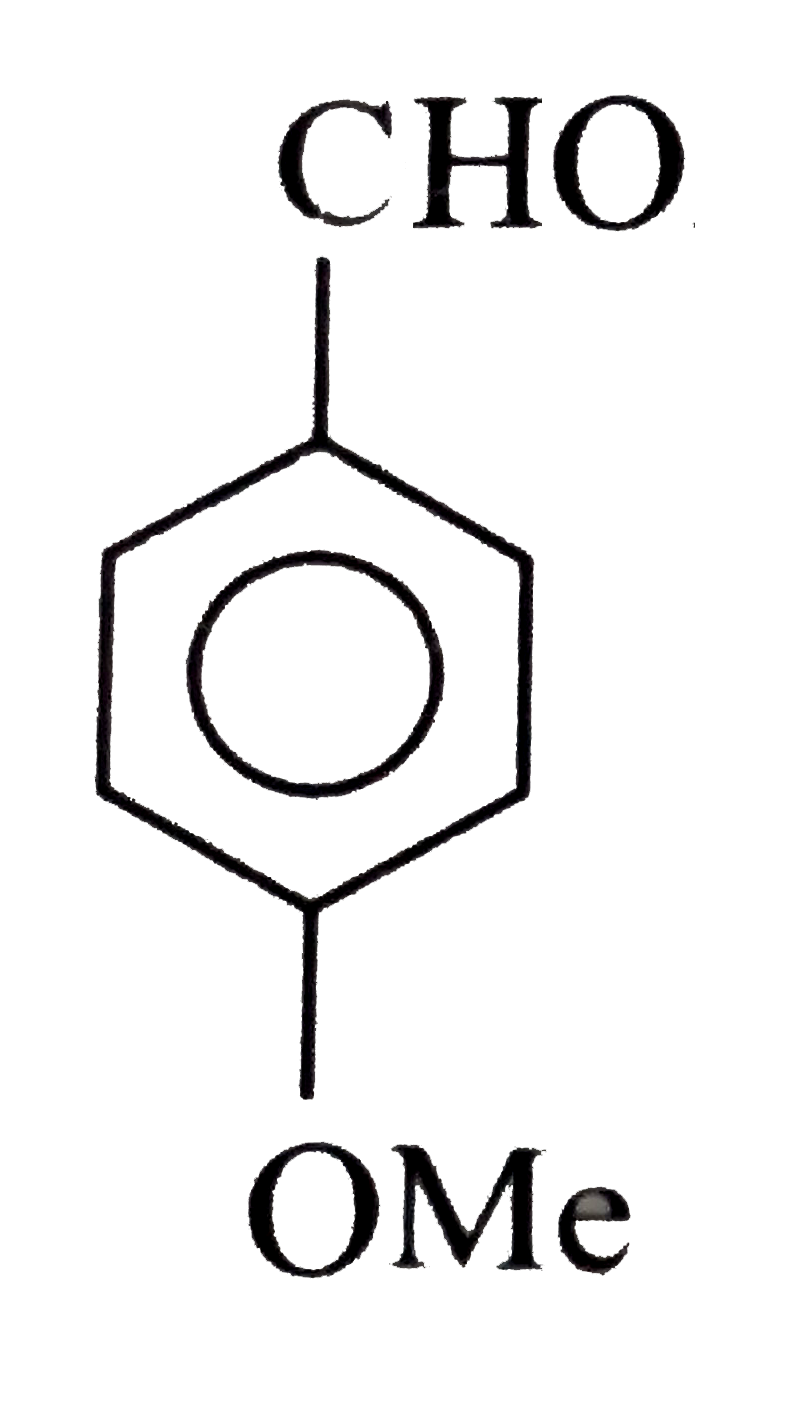
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|71 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|20 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Linked Comprehension
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- Reagent (P) is:
Text Solution
|
- Which of the statement is/are correct about compound (C )?
Text Solution
|
- Compound (D ) is:
Text Solution
|
- Compound (E ) is:
Text Solution
|
- Compound (A) is:
Text Solution
|
- Compound (B) is:
Text Solution
|
- Compound (C ) is:
Text Solution
|
- Reagent (D) is:
Text Solution
|
- Reagent (E ) is:
Text Solution
|
Text Solution
|
- Compound (G) is:
Text Solution
|
- Compound (H) is:
Text Solution
|
- Compound (B) is:
Text Solution
|
- Compound (C ) is:
Text Solution
|
- Reagent (D) is:
Text Solution
|