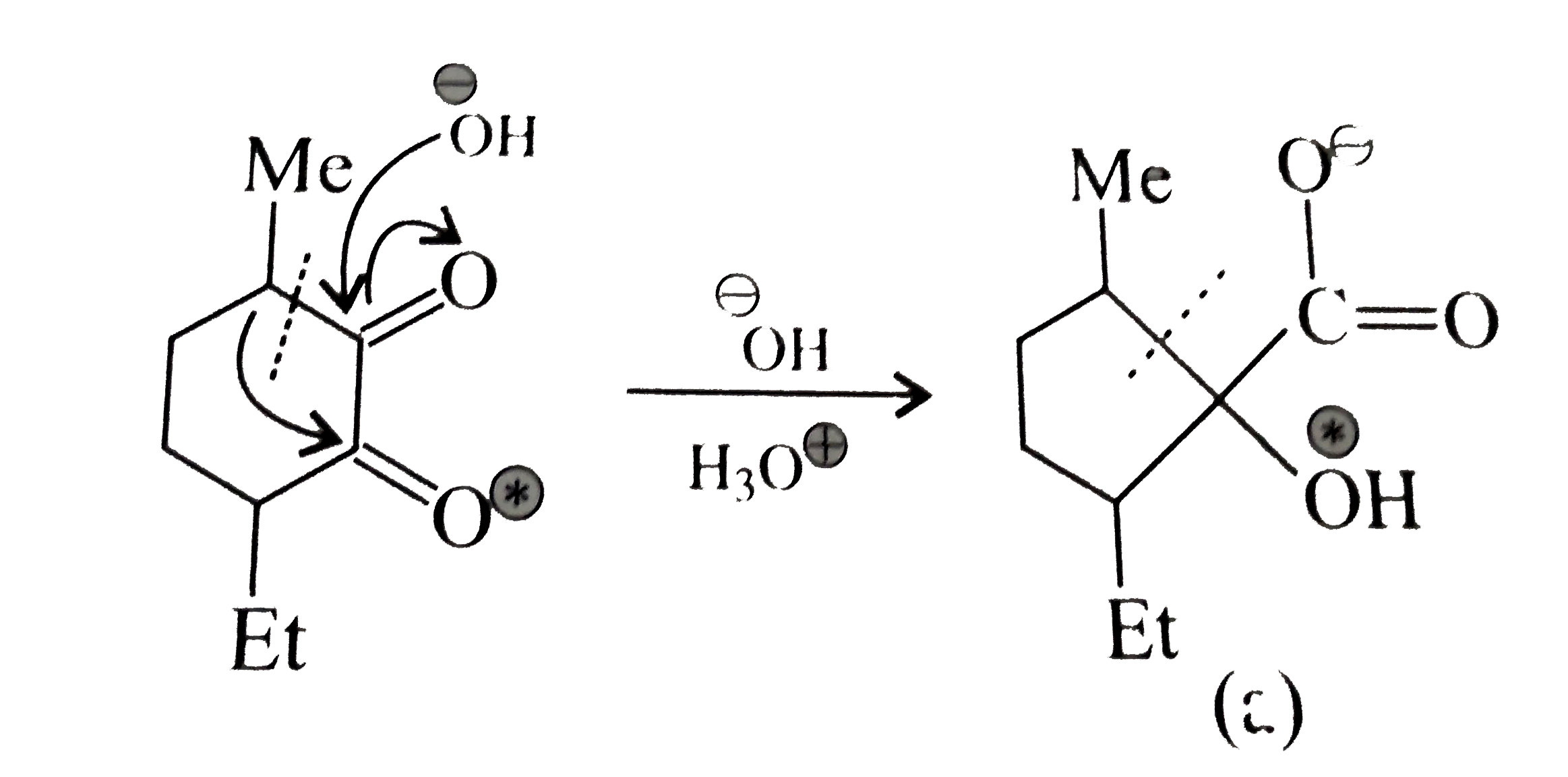
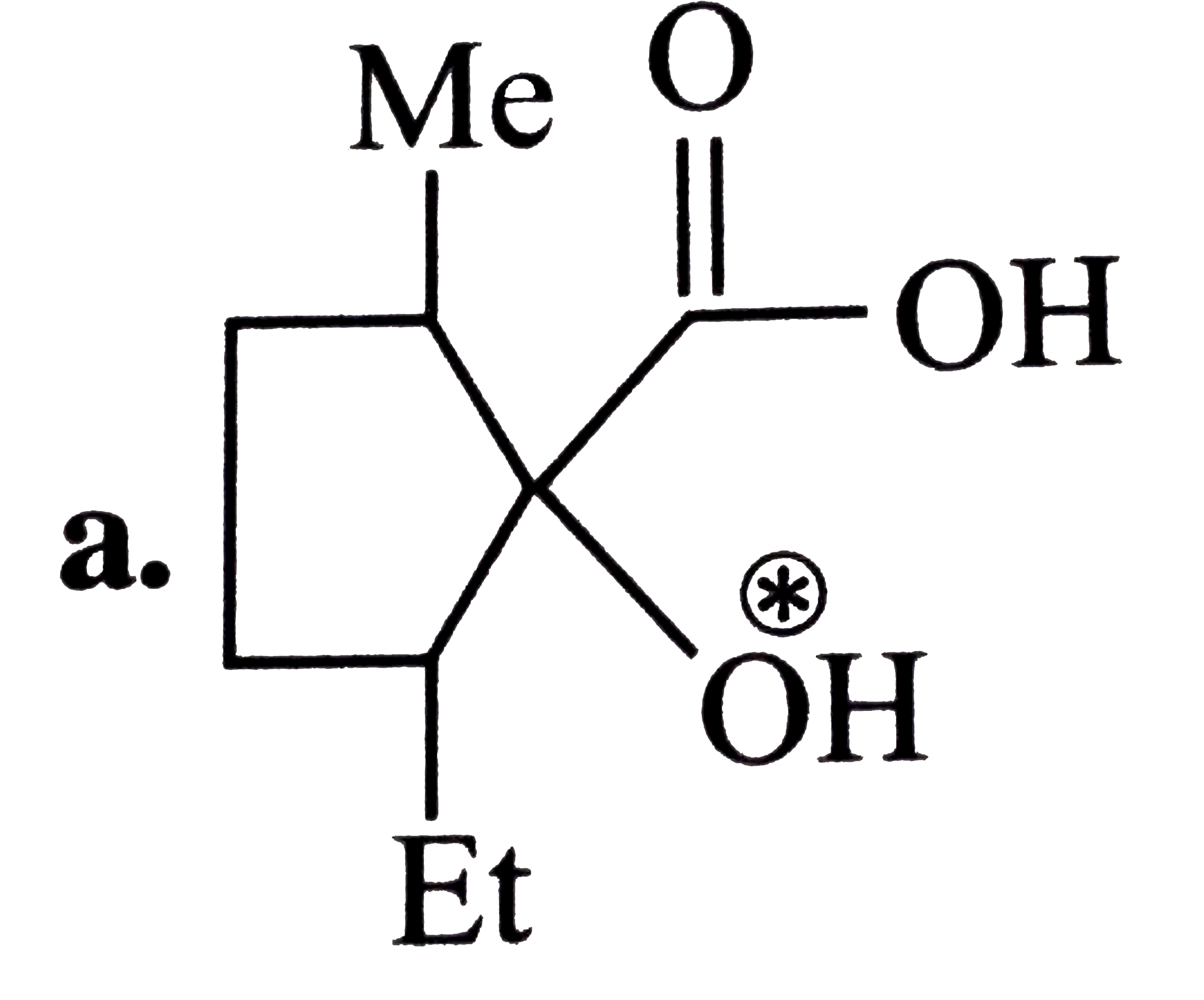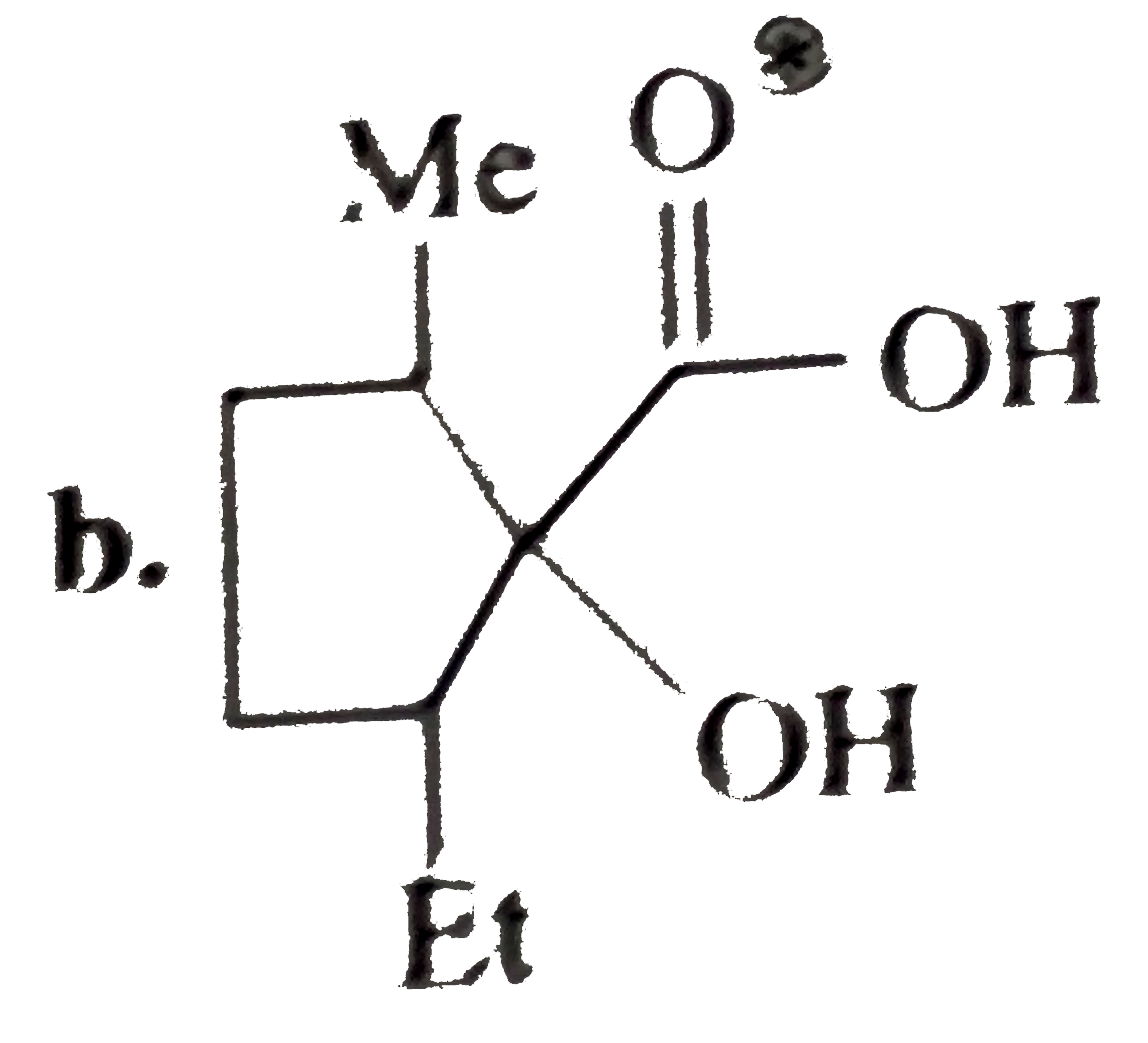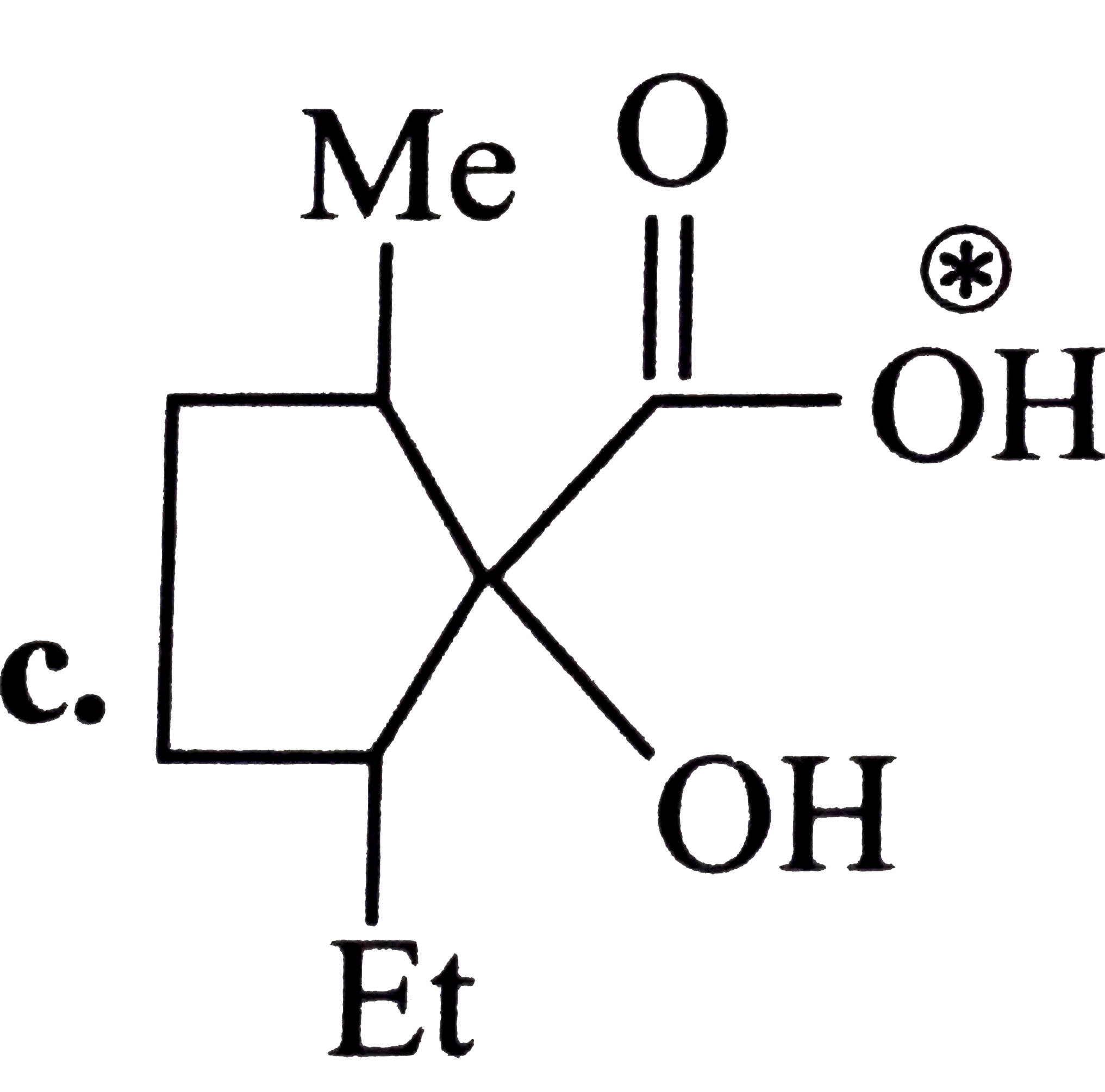A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|6 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Objective|10 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Single Correct
- State True or False. Evolution of gas is not a characteristic of chem...
Text Solution
|
- Me - -=-H overset(NaNH(2))underset(+liq.NH(3))rarr (C ) overset(H(3)O^...
Text Solution
|
- Compound (B) is:
Text Solution
|
- overset("High conc. of base")rarr ? + ? The products are:
Text Solution
|
- The products are:
Text Solution
|
- For the Cannizzaro reaction in which of the following statements is tr...
Text Solution
|
- For the Cannizzaro reaction in which of the following statements is tr...
Text Solution
|
- Two moles of HCHO and 1 mol of PhCHO react with conc. NaOH. What are t...
Text Solution
|
- Two moles of HCHO and 1 mol of PhCHO react with conc. NaOH. What are t...
Text Solution
|
- Identify the following disproportionation reaction. I. 2H-overset(D)...
Text Solution
|
- Aldehydes with alpha-H atom do not undergo disproportionation because:
Text Solution
|
- In Cannizzaro reaction, which of the following is a better hydride don...
Text Solution
|
- underset("donor")underset("Hydride")(RCHO) + underset("Acceptor")under...
Text Solution
|
- Identify the type of reaction (Exothermic/Endothermic) Respiration Ev...
Text Solution
|
- (A) undergoes Cannizzaro reaction. The products are:
Text Solution
|
- 2(A)overset(OH^(o+))rarr (B) + (C ). A is The product (C ) finds ap...
Text Solution
|
- Hydrogen gas is not released when Zn is treated with dilute Nitric aci...
Text Solution
|
- Which of the following sequence of reagents is correct in the conversi...
Text Solution
|
- Which method is feasible for the prepration of compound (X) only ?
Text Solution
|
- The different reagents are given as: (U) = NH(2)NH(2), (V) = H(3)O^(...
Text Solution
|
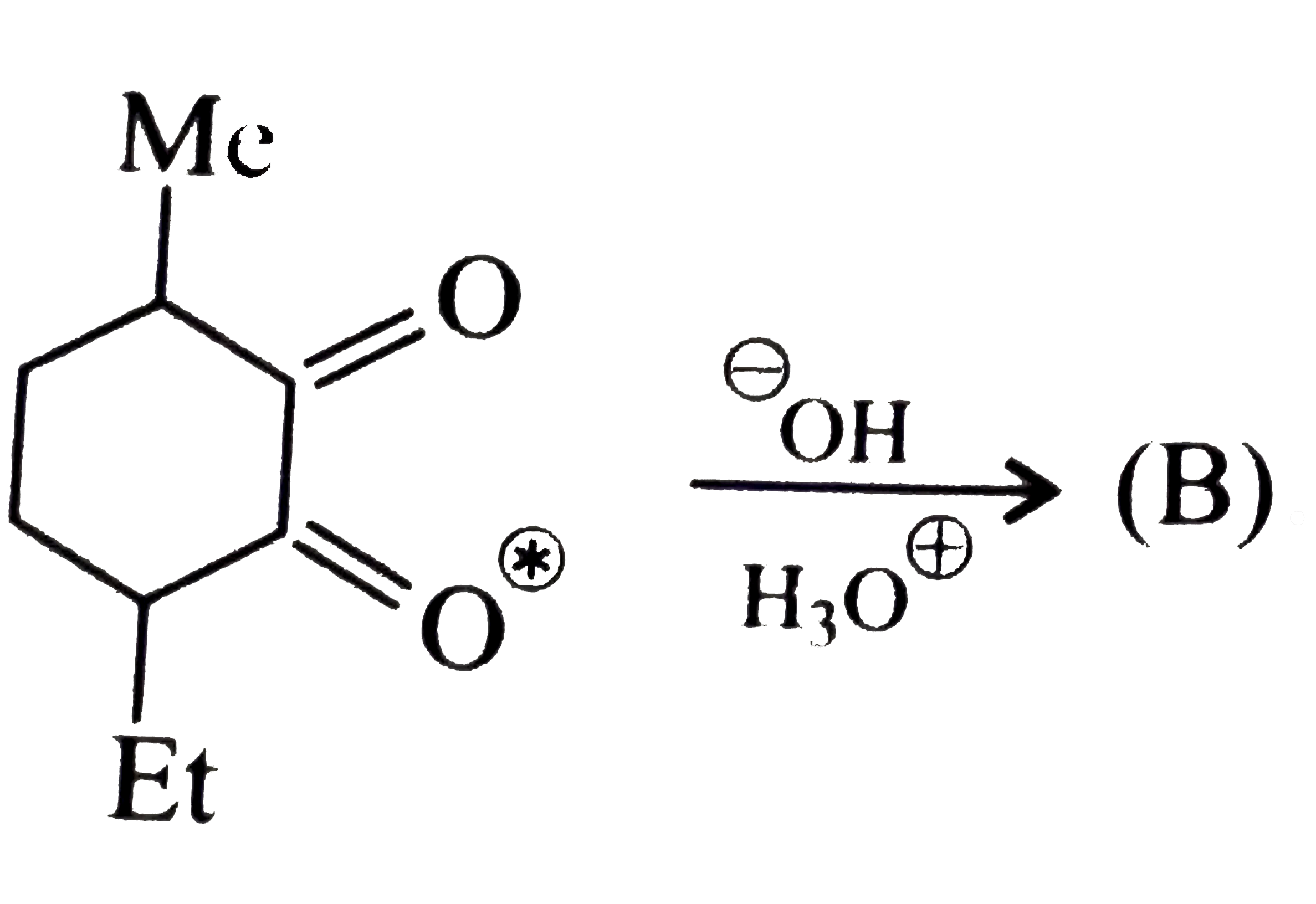 Compound (B) is:
Compound (B) is: