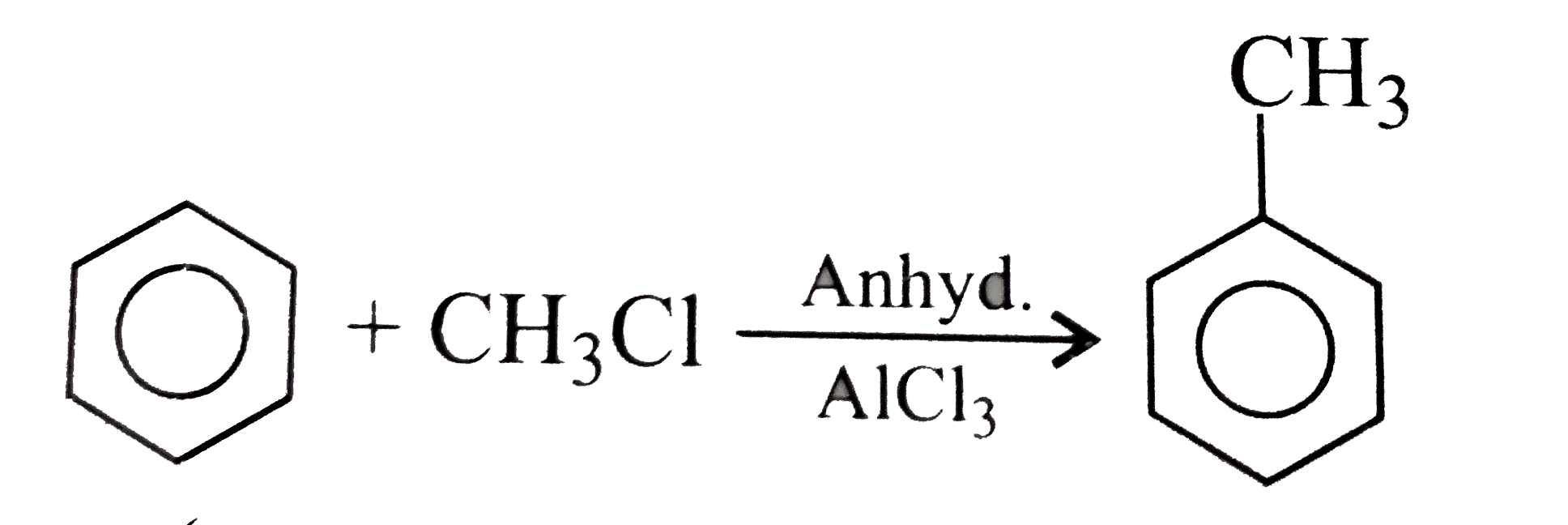A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In Theblanks|2 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives True And False|1 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Objective|10 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Archives Multiple Correct
- Base catalysed aldol condensation occurs with:
Text Solution
|
- Which of the following compounds will reacts with ethanolic KCN ?
Text Solution
|
- Which of the following are examples of aldol condensation ?
Text Solution
|
- A new carbon-carbon bond formation is possible in:
Text Solution
|
- Among the following compounds, which will react with acetone to give a...
Text Solution
|
- Which of the following will undergo aldol condensation ?
Text Solution
|
- Which of the following reactants on reaction with conc. NaOH followed ...
Text Solution
|
- 1^("st") member of ketone family and its next homologue reacts with NH...
Text Solution
|