Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-A Objective type Questions (One Option is correct))|45 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-B Objective type Questions (One Option is correct))|13 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J Aakash Challenger Questions )|10 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - J Aakash Challengers Questions)|6 VideosELECTROCHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - J) (Aakash Challengers Questions)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-COORDINATION COMPOUNDS -Try Yourself
- What is the coordination number of Co in [Co(NH(3))(4)(H(2)O)Br](NO(3)...
Text Solution
|
- What is the oxidation number of Fe in K(3)[Fe(C(2)O(4))(3)] ?
Text Solution
|
- The value of the reaction quotient Q for the cell Zn(s)∣Zn^(2+)(0.01M)...
Text Solution
|
- Calculate the emf of the given cell: Cu/Cu^(2+)(0.01M)∣∣Cu^(2+)(0.001M...
Text Solution
|
- Are the bidentate ligands same as the ambidentate ligands ?
Text Solution
|
- Write the formulas for the following coordination compounds : (1) Di...
Text Solution
|
- Write the IUPAC names of the following coordinates compounds : (1)...
Text Solution
|
- Are the following two compounds optical isomers ?
Text Solution
|
- Give evidence that [Co(NH(3))(5)Cl]SO(4) and [Co(NH(3))(5)(SO(4))]Cl a...
Text Solution
|
- Predict the hybridisation and geometry of [CoCl(4)]^(2-) and [Co(CN)(4...
Text Solution
|
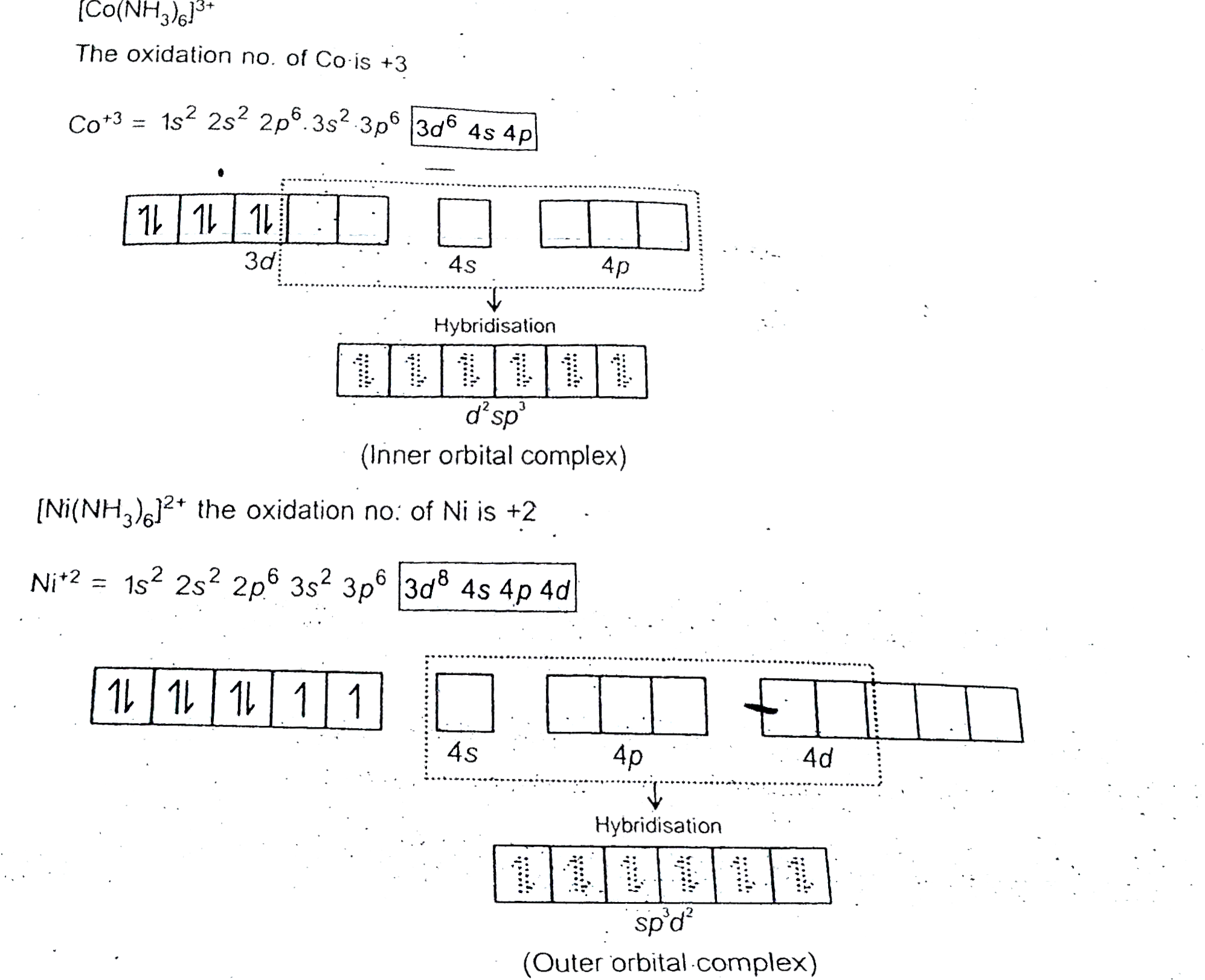
- Explain [Co(NH(3))(6)]^(3+) is an inner orbital complex whereas [Ni(NH...
Text Solution
|
- Assertion : [Fe(H(2)O)(6)]^(3+) is strongly paramagnetic whereas [Fe(C...
Text Solution
|
- Hexacyano complexes of metals in their + 2 oxidation state are usually...
Text Solution
|
- Which of the following is more stable ? (1) K(4)[Fe(CN)(6)] or K(3...
Text Solution
|