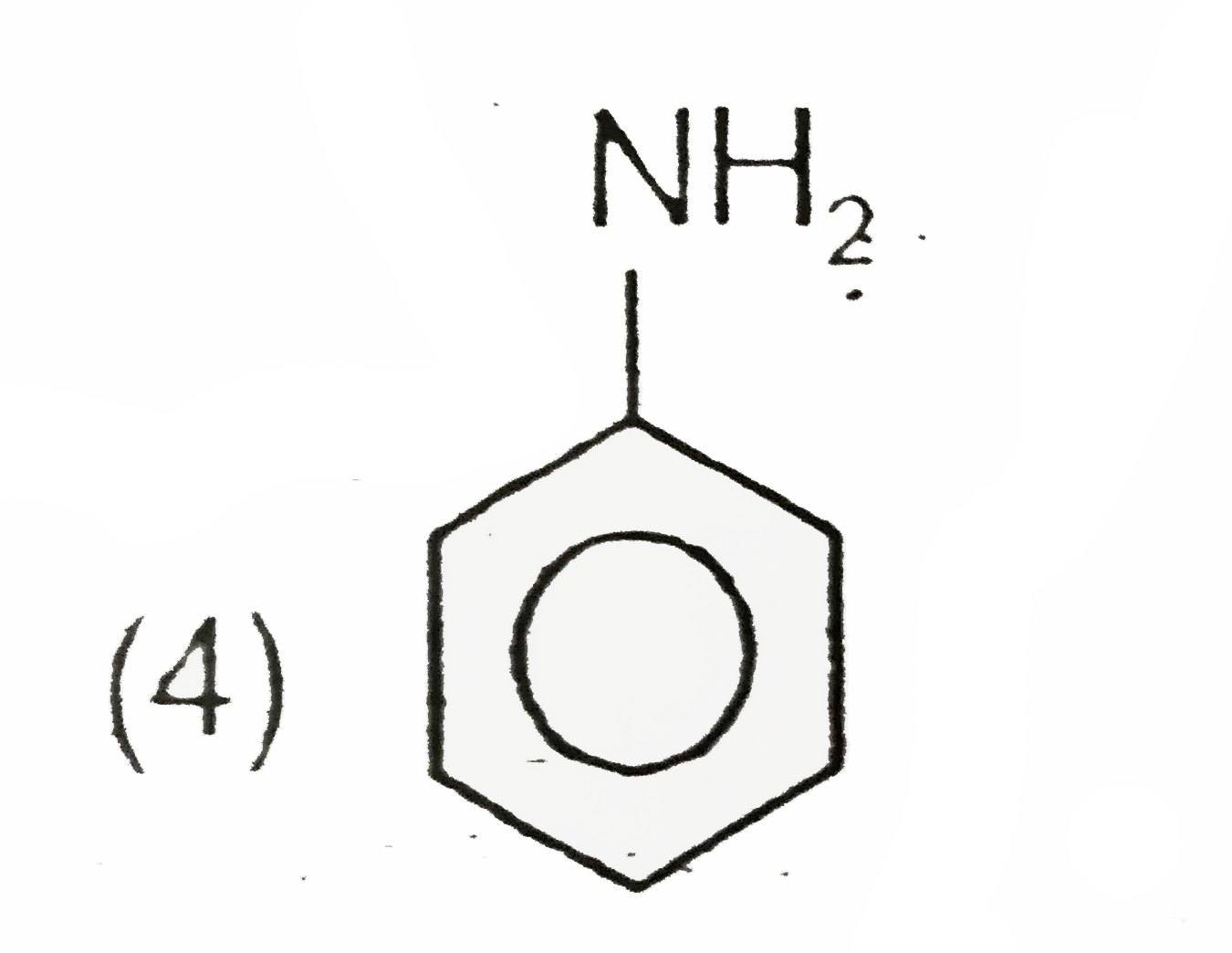
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section B (Objective Type Questions) one option is correct|23 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section C (Objective type questions more than one options are correct)|12 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|10 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|20 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-AMINES -Assignment Section -A Competition Level Questions
- The decreasing order of the basic character of NH(3), CH(3)NH(2), C(2)...
Text Solution
|
- The one which is the least basic
Text Solution
|
- Strongest base is
Text Solution
|
- An amine reacts with C(6)H(5)SO(2)Cl and the product is soluble in alk...
Text Solution
|
- Which one is most volatile?
Text Solution
|
- An organic compound (A) on reduction gave a compound (B). Upon treatme...
Text Solution
|
- The strongest ortho-para and strongest meta directing groups are respe...
Text Solution
|
- Which would not react with benzene sulphonyl chloride in aqueous NaOH?
Text Solution
|
- Aniline on reaction with acetyl chloride gives
Text Solution
|
- On heating an aliphatic primary amine with chloroform and ethanolic po...
Text Solution
|
- Which of the following compound gives dye test ?
Text Solution
|
- The action of nitrous acid on an aliphatic primary amine gives
Text Solution
|
- The gas evolved when methylamine reacts with nitrous acid is….
Text Solution
|
- Which of the following amines will form stable doazonium salt at 273-2...
Text Solution
|
- A mixture of 1^(@), 2^(@) and 3^(@) amines can be separated by Hinsber...
Text Solution
|
- Explain the following: Aniline readily reacts with bromine to give ...
Text Solution
|
- CH3CH2Cloverset(NaCN)to Xoverset(Ni//H2)to Yunderset("anhydride")overs...
Text Solution
|
- Identify X in the series
Text Solution
|
- A compound 'A' when treated with HNO(3) (in presence of H(2)SO(4)) giv...
Text Solution
|
- A secondary amine is a compound which possesses .
Text Solution
|