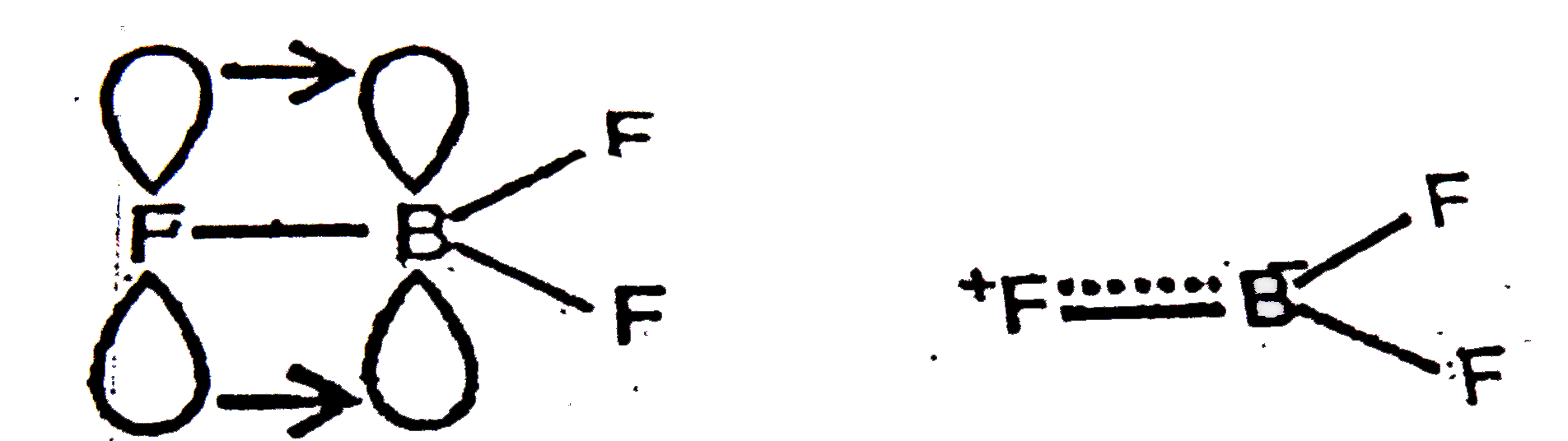A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise Example|30 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise B.L.E|49 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P BLOCK ELEMENTS-INORGANIC CHEMISTRY(P-Block Elements)
- Nitrogen and oxygen exist as diatomic but their congeners are P(4) and...
Text Solution
|
- H(3)BO(3) is.
Text Solution
|
- The Lewis acid character of halides of boron are as follows :
Text Solution
|
- Ammonia can be dried by :
Text Solution
|
- Xe F(6) on completely hydrolysis gives
Text Solution
|
- Which of the following metals gives N(2)O gas with dilute HNO(3) ?
Text Solution
|
- HNO(3) on dehydration with phosphorus pentaoxide yields :
Text Solution
|
- Holme's signals can be given using
Text Solution
|
- How can the following reaction be made to proceed in forward direction...
Text Solution
|
- What are the products formed in the reaction of xenon hexafluoride wit...
Text Solution
|
- Which of the following compounds gives a mixture of SO(2) and SO(3) ...
Text Solution
|
- Which oxide of carbon is obtained when K(4)[Fe(CN)(6)] is warmed with...
Text Solution
|
- Which of the following options correctly describes the reagents , prod...
Text Solution
|
- Select the incorrect order .
Text Solution
|
- Which of the following statements about pH and H^(+) ion concentration...
Text Solution
|
- Which of the following statements in incorrect ?
Text Solution
|
- Hydrolysis of one mole of peroxodisulphuric acid produces
Text Solution
|
- The threshold wavelength for ejection of electrons from a metal is 430...
Text Solution
|
- The number of photons of light of barv =4.5×10^6m^(−1) necessary to p...
Text Solution
|
- Which of the following on heating produces NO(2) ?
Text Solution
|