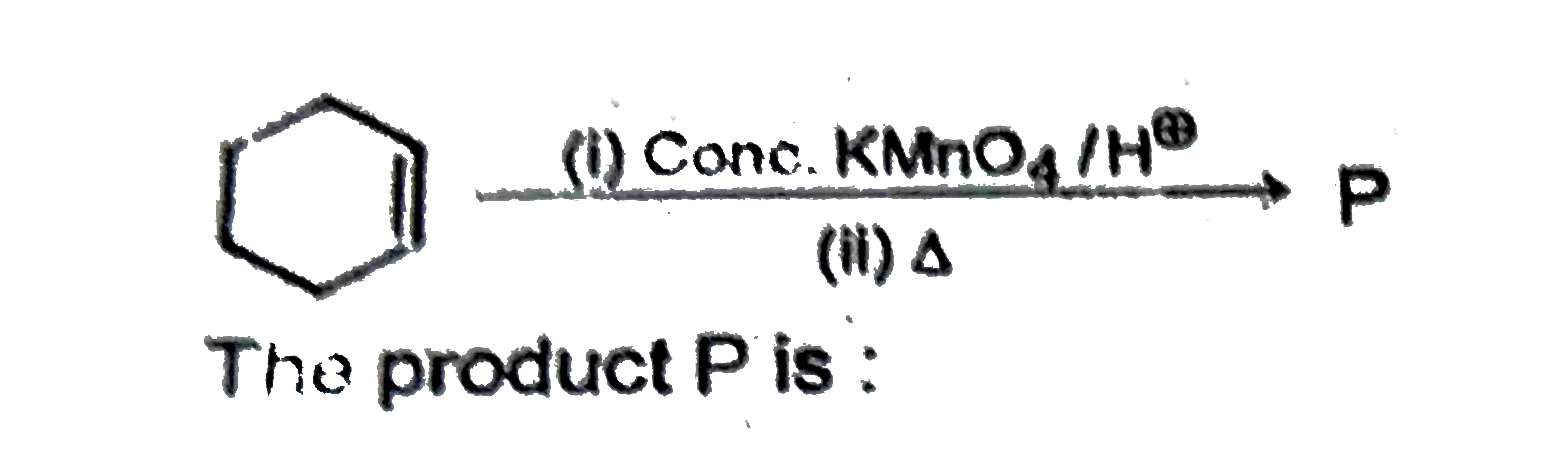A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES, CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|15 VideosALDEHYDES, KETONES, CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)|40 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ALDEHYDES, KETONES, CARBOXYLIC ACID-ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
- If the Planck's constant h=6.6×10^(-34) Js, the de Broglie wave length...
Text Solution
|
- A new carbon-carbon bond formation is possible in:
Text Solution
|
- Aldehydes and ketones may be distinguished by using
Text Solution
|
- If the Planck's constant h=6.6×10^(-34) Js, the de Broglie wave length...
Text Solution
|
- If the Planck's constant h=6.6×10^(-34) Js, the de Broglie wave length...
Text Solution
|
- If the Planck's constant h=6.6×10^(-34) Js, the de Broglie wave length...
Text Solution
|
- The product P is :
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- How many electrons with l=2 are there in an atom having atomic number ...
Text Solution
|
- An atom has mass of 0.02 kg and uncertainty in its velocity is 9.218×1...
Text Solution
|
- An atom has mass of 0.01 kg and uncertainty in its velocity is 7.318×1...
Text Solution
|
- An atom has mass of 0.2 kg and uncertainty in its velocity is 6.218×10...
Text Solution
|
- CH(3)CH(2)CH(2)CH(2)CH(2)COOHrarrCH(2)CH(2)CH(2)-overset(overset(NH(2)...
Text Solution
|
- An atom has mass of 0.01 kg and uncertainty in its velocity is 4.318×1...
Text Solution
|