Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-2 Part-III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE|26 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-2 Part-IV : COMPREHENSIONS|11 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-2 Part-I : ONLY ONE OPTION CORRECT TYPE|26 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Exercise-2 Part-II : SINGLE AND DOUBLE VALUE INTEGER TYPE
- Central atom may exhibit sp^(3) hybridisation in how many of the follo...
Text Solution
|
- Mg+BtoMg(x)B(y)overset("HCl")toDiborane Report your answer as (x+y)...
Text Solution
|
- B+underset(conc.)(HNO(3))tounderset("white solid")(A)+underset("brown ...
Text Solution
|
- How many of the following order of bond energies are correct. (i) C...
Text Solution
|
- Which of the following Salts are amphoteric in anture. PbO,PbO(2), ...
Text Solution
|
- the summation of atomicity of compounds in x,y and z is?
Text Solution
|
- Reduction of 117.5 g BCl(3) by H(2) in silent electric discharge prod...
Text Solution
|
- 4 moles of NaBH(4) react completely with I(2). calculate volume of ...
Text Solution
|
- Total number of Boron atoms in anionic part of borax which participate...
Text Solution
|
- How many compounds show amphoteric nature amongst following B(2)O(3)...
Text Solution
|
- How many compounds form acidic solution when dissolved in water H(3)...
Text Solution
|
- B(10)C(2)H(12) is isostructural & isoelectronic with borate ion of for...
Text Solution
|
- No of compounds producing gas on hydrolysis (with H(2)O ) is Al(4)C(...
Text Solution
|
- How many of the given statements are true for potash alum. (1) it i...
Text Solution
|
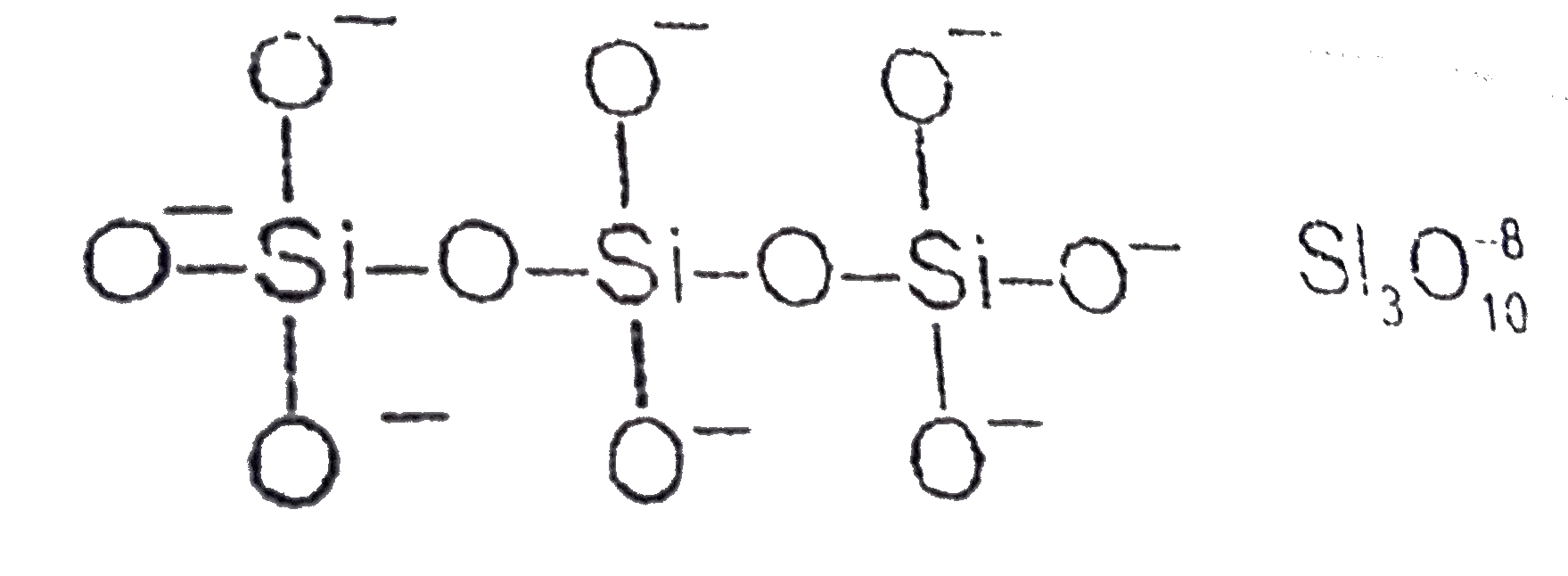
- Sum of the number of oxygen shared in between two silicon atoms in Si(...
Text Solution
|
- Number of oxygen atoms shared per SiO(4)^(4-) tetrahedron in single ch...
Text Solution
|
- the overall charge present on the cyclic silicate anion [Si(6)O(18)]^(...
Text Solution
|
- The silicate ion in the mineral kinoite is a chain of three SiO(4)^(4-...
Text Solution
|
- Straight chain polymer is formed by hydrolysis of [x] which is tetrasu...
Text Solution
|
- Zeolites are minerals of the composition M((x/n)) [[AlO2]x (SiO2)y].ZH...
Text Solution
|