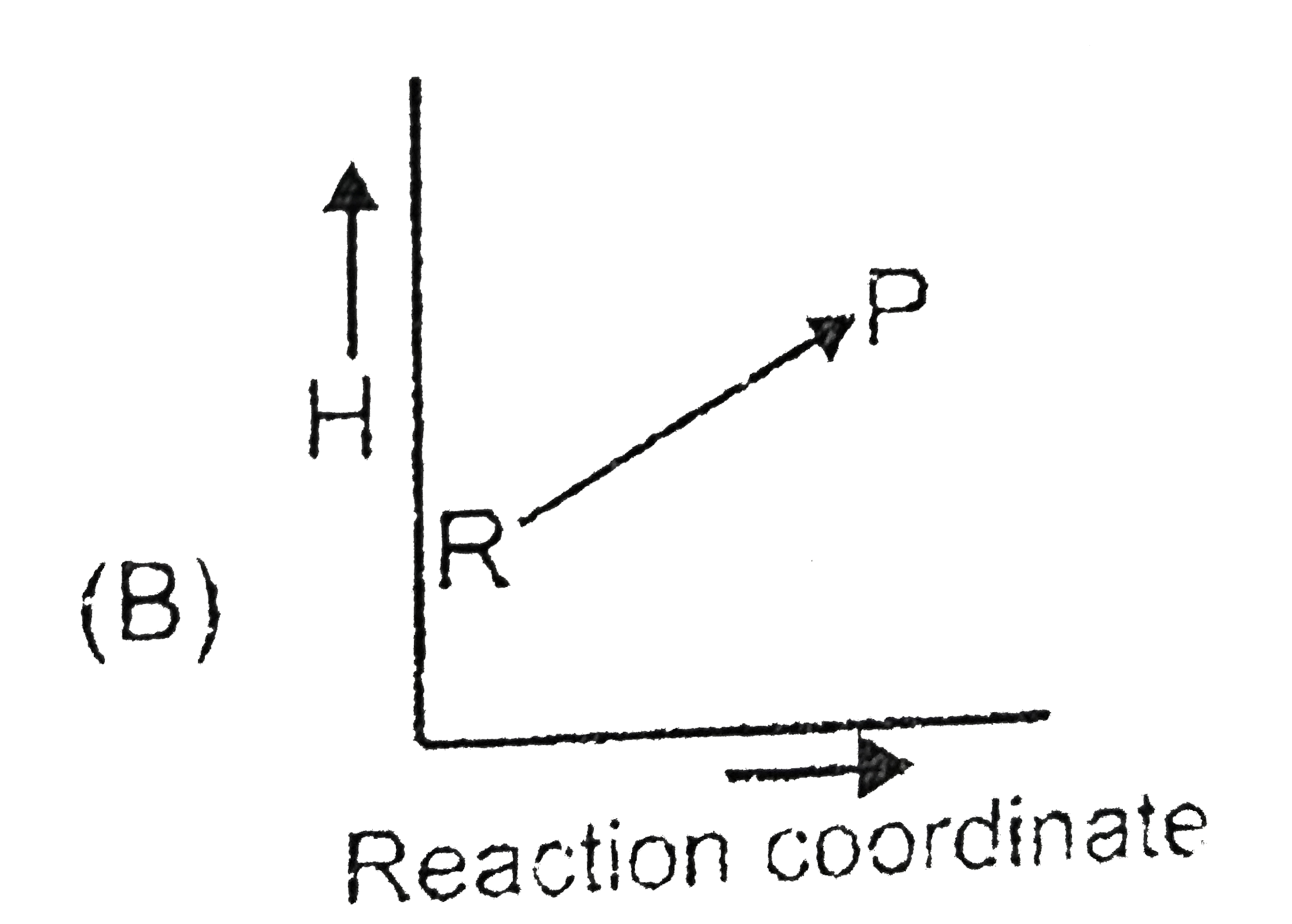
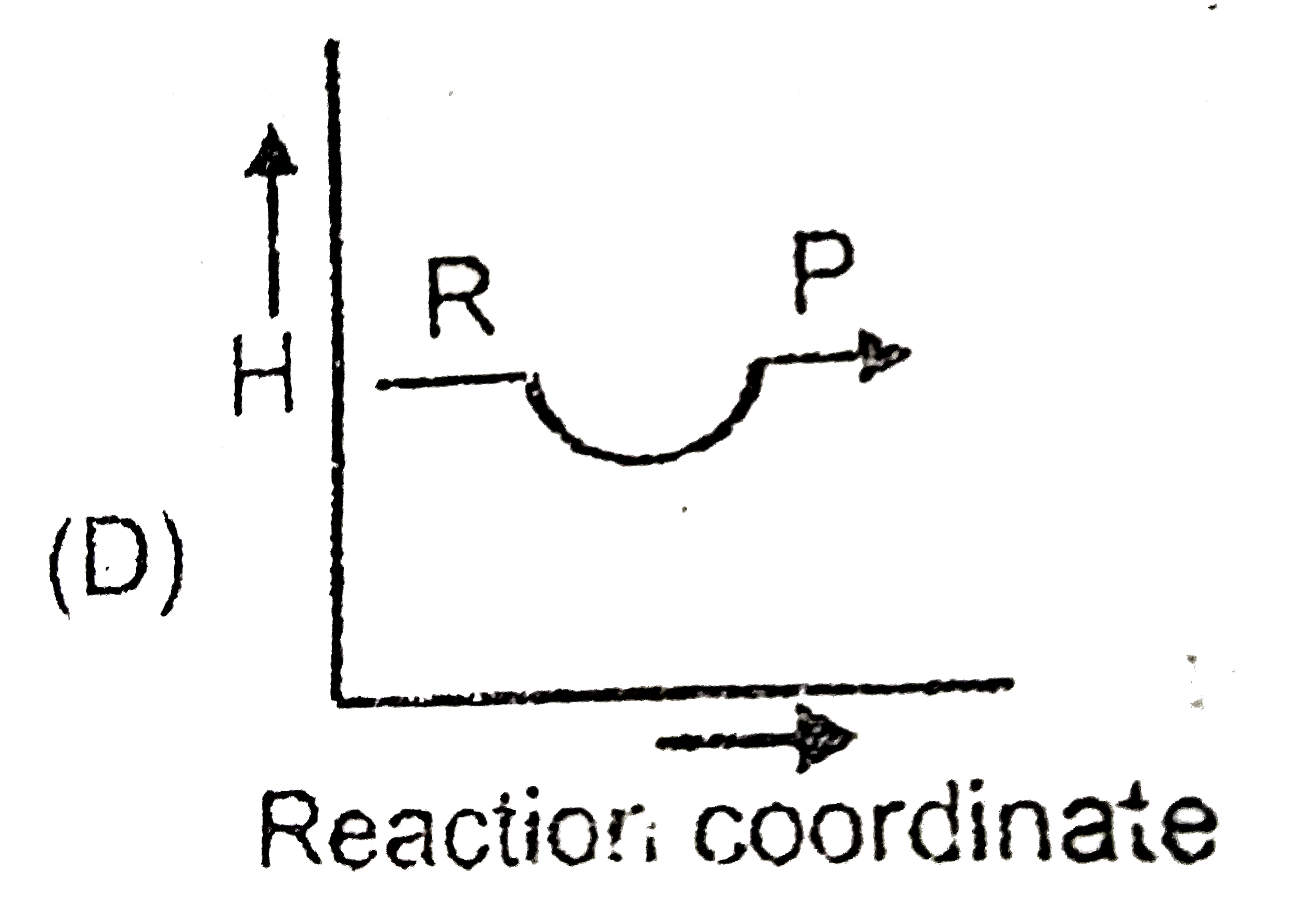
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise -2 Part-I: Only one option correct type|23 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-2 II: Single and double value integer type|16 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise H-1|1 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise -1 Part -II Only option correct type
- Consider the reaction at 300 K H(2)(g)+Cl(2)(g)rarr2HCl(g), DeltaH...
Text Solution
|
- For gaseous reactions, if DeltaH is the change in enthalpy and DeltaU ...
Text Solution
|
- Which plot represent an exothermic reaction ?
Text Solution
|
- For which of the following change Delta H!=Delta U?
Text Solution
|
- Calculate the standard internal energy of formation of liquid methyl a...
Text Solution
|
- 2C+O(2)rarr2CO, Delta H= - 220 kJ which of the following statements is...
Text Solution
|
- {:(C(s)+O(2)(g)rarrCO(2)(g), ,,,,DeltaH= -94.3 kcal//mol),(CO(g)+(1)/(...
Text Solution
|
- In the reaction, CO(2)(g)+H(2)(g)toCO(g)+H(2)O(g)," "DeltaH=2.8 ...
Text Solution
|
- Given, H(2)(g)+Br(2)(g)rarr2HBr(g),DeltaH(1)^(0) and standard enthalpy...
Text Solution
|
- Calculate the heat of transition for carbon from the following: C("D...
Text Solution
|
- The standard heat of combustion of a solid boron is equal to:
Text Solution
|
- The heat of combustion of sucrose (C(12)H(22)O(11)) is 1350 kcal//mol....
Text Solution
|
- If S+O(2)toSO(2),DeltaH=-298.2 " kJ" " mole"^(-1) SO(2)+(1)/(2)O(2)t...
Text Solution
|
- When a certain amount of ethylene was combusted, 5644 kJ heat was evol...
Text Solution
|
- AT 300K, the standard enthalpies of formation of C(6)H(5)COOH(s), CO(2...
Text Solution
|
- In the reaction CS(2)(l)+3O(2)(g)rarrCO(2)(g)+2SO(2)(g)DeltaH= -265 kc...
Text Solution
|
- If enthalpy of dissociation of CH(4) and C(2)H(6) are 320 and 360 calo...
Text Solution
|
- The heat of hydrogenation of ethane is x(1) and heat of benzene is x(2...
Text Solution
|
- Calculate the resonance energy of gaseous benzene from the given data ...
Text Solution
|
- Bond dissociation energies of H(2),Cl(2) and HCl((g)) are 104, 58 and ...
Text Solution
|