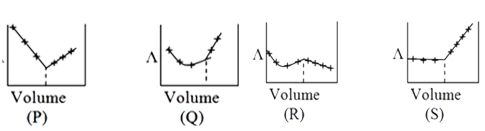
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Exercise 3|7 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Part 2|23 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Objective Questions|41 VideosELECTRO CHEMISTRY
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (ELECTROCHEMISTRY)|53 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Part -IV|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTROCHEMISRY-Comprehension
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- Strong acid versus strong base: The principle of conductometric titr...
Text Solution
|
- The graph represents the titration curve for : a.Strong acid and stro...
Text Solution
|
- Tollen's reagent is used for the detection of aldehyde when a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- We have taken a saturated solution of AgBrK(sp) of AgBr is 12 xx 10^(-...
Text Solution
|
- Chemical reaction involve interaction of atoms and molecules. A large ...
Text Solution
|
- If the cathode is a Hg electrode, the maximum weight (g) of amalgam fo...
Text Solution
|
- Chemical reaction involve interaction of atoms and molecules. A large ...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- For the reduction of NO(3)^(c-) ion in an aqueous solution, E^(c-) is ...
Text Solution
|
- A simple model for a concentration cell involving a metal M is M(s)|...
Text Solution
|
- A simple model for a concentration cell involving a metal M is M(s)|...
Text Solution
|
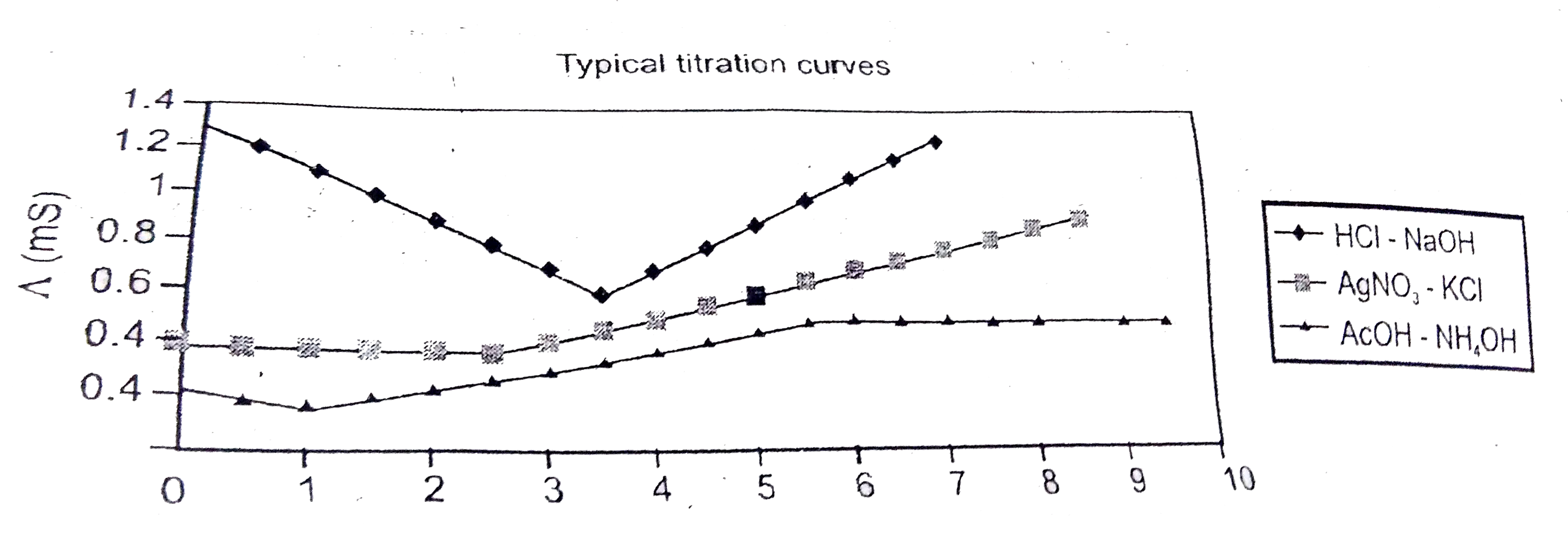
- AgNO(3)(aq) was added to an aqueous KCl solution gradually and the con...
Text Solution
|
- Consider the following cell reaction 2Fe((s))+O(2(g))+4H((aq.))^(o+)...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M|M^(2+...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M|M^(2+...
Text Solution
|