Text Solution
Verified by Experts
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-II ONLY ONE OPTION CORRECT TYPE|12 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Section-B Nitrogen containing compounds|14 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Section B|7 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS -Section C
- The final product of the following reaction is:
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- In a reaction of aniline a coloured products C was obtained.
Text Solution
|
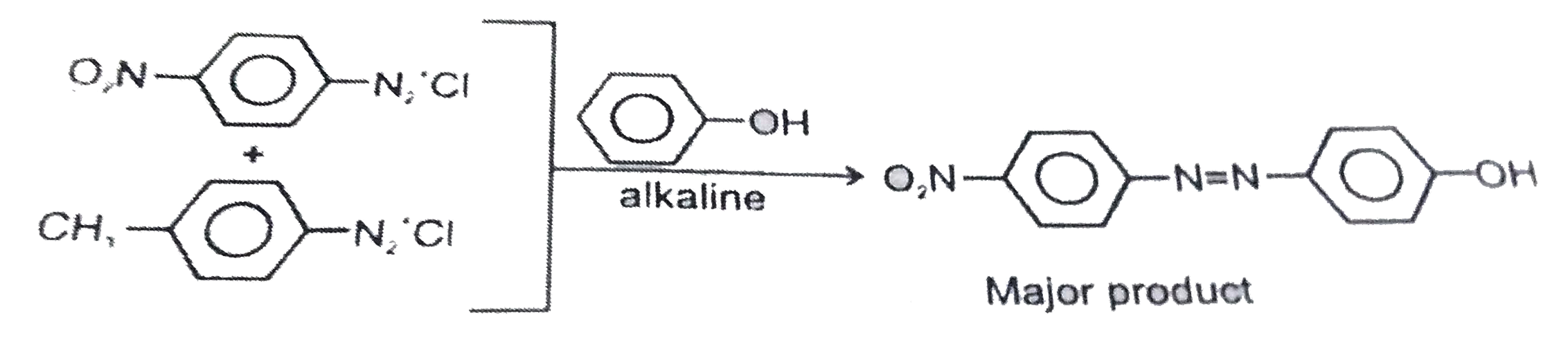
- A solution contains 1g mol. Each of p-toluene diazonium chloride and p...
Text Solution
|
- How will you carry out the follwing conversion? (i) Toluene -p-tolui...
Text Solution
|