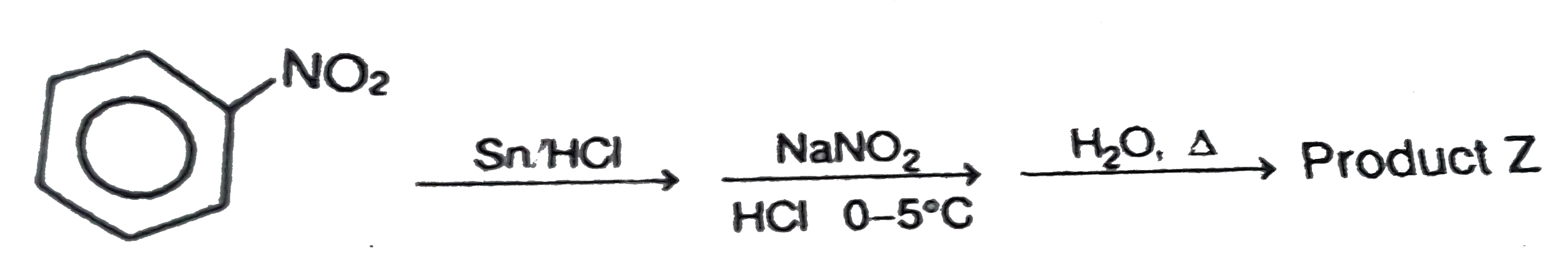Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-III ONE OR MORE THAN ONE OPTION CORRECT TYPE|10 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART IV- COMPREHENSION|13 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise -2 Part-I only one option correct type|11 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS -PART-II SINGLE OR DOUBLE INTEGER TYPE
- Identify molecular weight of final product (Y).
Text Solution
|
- How many toluidines on reaction with NaNO(2)//HCl followed by H(3)PO(2...
Text Solution
|
- Find the molecular weight of Z.
Text Solution
|
- Molecular weight of T will be:
Text Solution
|
- How many N atom are present in final product.
Text Solution
|
- Find the molecular weight of Y report your answer as ("Molecular weigh...
Text Solution
|
- In the given reaction how many of the following products (1-9) can be ...
Text Solution
|