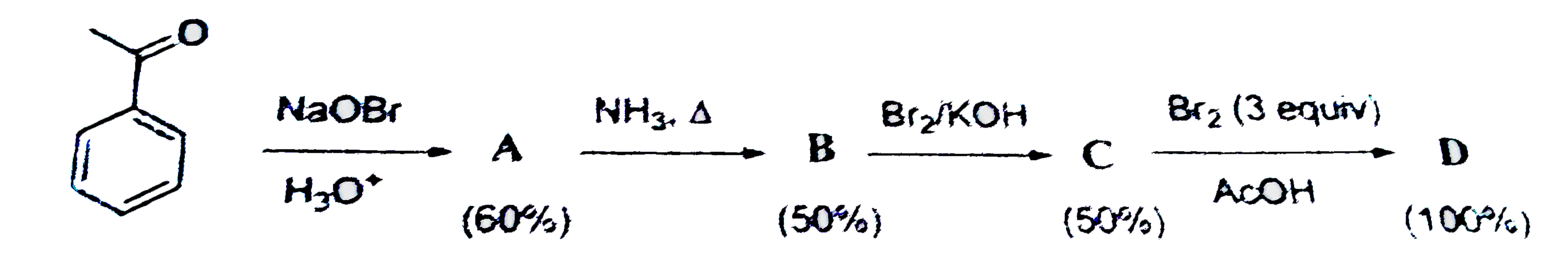
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - II JEE MAIN|20 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise JEE MAIN (Online Problem)|15 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - IV Comprehension|13 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS-Exercise-3 (Part-I) JEE Advanced
- Match the transformation in Column I with appropriate option in Column...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- Amongst the compounds given, the one that would form a brilliant color...
Text Solution
|
- In the following reaction, the product (s) formed is (are)
Text Solution
|
- The major product of the following reaction contain x bromine atoms in...
Text Solution
|
- P and Q are isomers of dicarboxylic acid C(4)H(4)O(4). Both decolorize...
Text Solution
|
- The reactivity of compound Z with different halogens under appropriat...
Text Solution
|
- For the identification of beta-napthtol using dye test, it is necessar...
Text Solution
|
- Match the four material (P, Q, R, S) given in List -I with the corresp...
Text Solution
|
- Among the following, the number of reaction(s) that produce(s) benzal...
Text Solution
|
- The major product U in the following reactions is
Text Solution
|
- In the following reactions, the major product W is:
Text Solution
|
- The product (s) of the following reaction sequence is (are)
Text Solution
|
- The correct statement(s) about of the following reaction sequence is(a...
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- The reaction of compounf P with CH(3)MgBr (excess) in (C(2)H(5)(2)O fo...
Text Solution
|
- The reaction of compounf P with CH(3)MgBr (excess) in (C(2)H(5)(2)O fo...
Text Solution
|
- The reaction(s) leading to the formation of 1,35- trimehtylbenzene is ...
Text Solution
|
- Aniline reacts with mixed acid (conc. HNO(3) and conc. H(2)SO(4)) at 2...
Text Solution
|
- In the following reaction sequence, the amount of D (in g) formed from...
Text Solution
|