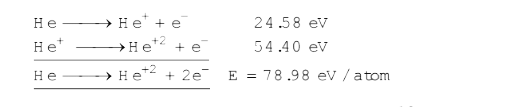Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -1|75 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -2|50 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -JEE ADVANCE (ARCHIVE)
- The first and second ionization potentials of helium atoms are 24.58 e...
Text Solution
|
- The energy released when an electron is added to a neutral gaseous ato...
Text Solution
|
- The hydration energy of Mg^(+2) is greater than that of
Text Solution
|
- Arrange the following in the given order (a) Decreasing ionic size, ...
Text Solution
|
- On Mulliken scale, the average of ionisation potential and electron af...
Text Solution
|
- The softness of group IA metals increase down the group with increasin...
Text Solution
|
- Compare qualitatively the first and second ionisation potentials of co...
Text Solution
|
- Arrange the following in the order of their increasing size : Cl^(-) ,...
Text Solution
|
- In group IA of alkali metals, the ionisation potential decrease down t...
Text Solution
|
- The electronegativity of the following elements increases in the order
Text Solution
|
- Atomic radii of fluorine and neon (Å) respectively are given as
Text Solution
|
- The first ionisation potential (in electron volts) of nitrogen and oxy...
Text Solution
|
- The first ionisation enthalpies of Na, Mg , Al and Si are the order
Text Solution
|
- Which of the following statements is/are true for the long form of the...
Text Solution
|
- The first ionisation potential (in electron volts) of nitrogen and oxy...
Text Solution
|
- Which one of the following is the smallest in size?
Text Solution
|
- Why the first ionisation energy of carbon atom is greater than that of...
Text Solution
|
- Amongst the element with following electronic configurations, which on...
Text Solution
|
- Arrange the following as stated: Increasing order of ionic size N^(3...
Text Solution
|
- The statement that is not correct for periodic classification of eleme...
Text Solution
|
- Ca^(2+) the first ionisation energies of elements along a period do no...
Text Solution
|