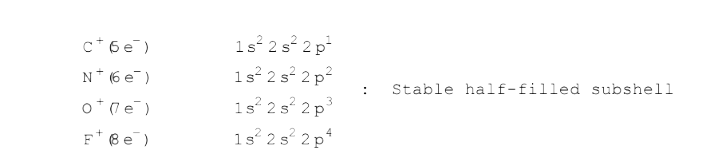A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE)|30 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -2 Numerical Value Type for JEE Main|13 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -JEE MAIN (ARCHIVE)
- The correct order of second ionisation potentials of carbon, nitrogen,...
Text Solution
|
- The element with the highest first ionization potential is:
Text Solution
|
- The correct order of radii is
Text Solution
|
- The first ionisation potential of Na is 5.1eV. The value of electron g...
Text Solution
|
- Which of the following represents the correct order of increasing firs...
Text Solution
|
- The ionic radii (in Å) of N^(3-), O^(2-) and F^(-) are respectively :
Text Solution
|
- Which one has the highest boiling point ?
Text Solution
|
- Statement I- Nitrogen and oxygen are the main components in the atmosp...
Text Solution
|
- The isoelectronic set of ions is:
Text Solution
|
- The element having greatest difference between its first and second io...
Text Solution
|
- In isoelectronic species Cl^(-),Ar,Ca^(2+) size differ due to
Text Solution
|
- In comparison to B, Be has
Text Solution
|
- The IUPAC symbol for the element with atomic number 119 would be:
Text Solution
|