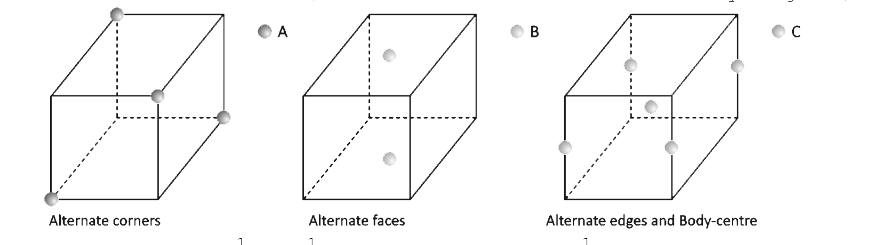
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2 (Numerical Value Type for JEE Main )|15 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise JEE Main (Archive )|39 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-LEVEL-2
- In the fluorite structure if the radius ratio is (sqrt(3/(2))-1) how m...
Text Solution
|
- Following three planes (P1, P2, P3) in an FCC unit cell are shown. Con...
Text Solution
|
- In a cubic,A atoms are present on alternative corners, B atoms are pre...
Text Solution
|
- For the spinel structure (MgAl(2)O(4)), the correct statement is//are
Text Solution
|
- In a unit cell, atoms (A) are present at all corner lattices, (B) are ...
Text Solution
|
- In a unit cell, atoms (A) are present at all corner lattices, (B) are ...
Text Solution
|
- In a unit cell, atoms (A) are present at all corner lattices, (B) are ...
Text Solution
|
- If the lattice parameter of Si = 5.43 Å and the mass of Si atom is 28....
Text Solution
|
- The lacttice parameter of GaAs (radius of Ga = 1.22 Å, As = 1.25 Å) is
Text Solution
|
- In cubic ZnS (II-VI) compounds, if the radii of Zn and S atoms are 0.7...
Text Solution
|
- An elemental crystal has density of 8570 kg m^(-3). The packing effici...
Text Solution
|
- A metal (M^(@) = 100 gm/mole) having density 10 gm/cm^(3) forms a cubi...
Text Solution
|
- Every atom or ion that forms an fcc unit cell is surrounded by
Text Solution
|
- Consider the structure of CsCl (8: 8 coordination). How many Cs^(o+) i...
Text Solution
|
- A metal of density 7.5 xx 10^(3) kg m^(-3) has an fcc crystal structur...
Text Solution
|
- The ratio of the volume of a tetragonal lattice unit cell to that of a...
Text Solution
|
- An fcc lattice has a lattice parameter a = 400 pm. Calculater the mola...
Text Solution
|
- A TV in fcc is formed by atoms at
Text Solution
|
- What is the density of Na(2)O having antifluorite-type crystgal stryct...
Text Solution
|
- If the radious of Cs^(o+) = 1.69 Å and Br^(Θ) = 1.95 Å, then which of ...
Text Solution
|