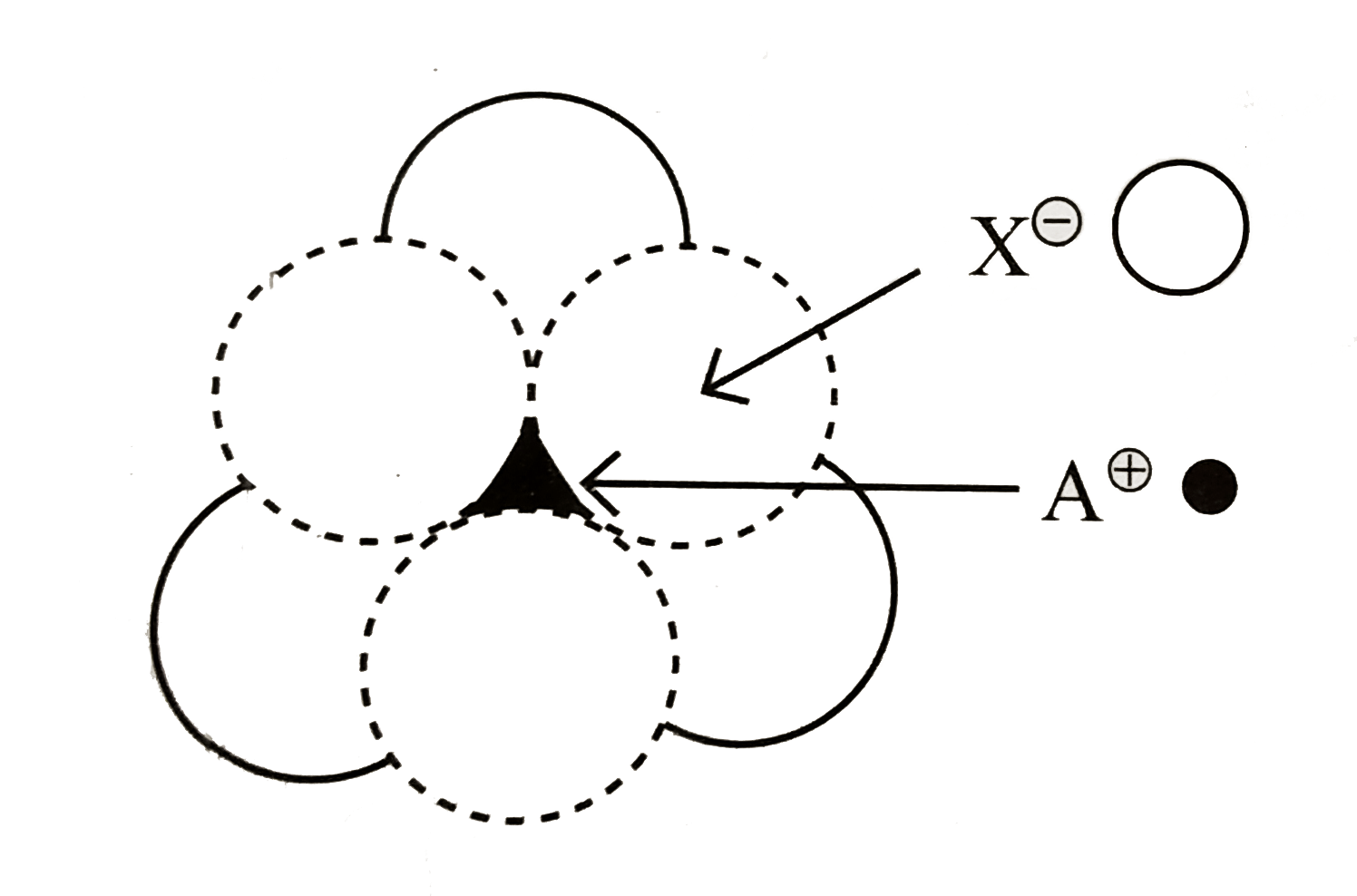
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise FUNDAMENTAL|50 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise ENABLE|50 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise JEE Main (Archive )|39 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-JEE Advance ( Archive )
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- A substance A(x)B(y) crystallizes in a face-centred cubic lattice in ...
Text Solution
|
- You are given marbles of diameter 10mm. They are to be placed such tha...
Text Solution
|
- The crystal AB (rock salt structure) has molecular weight 6.023 y u, w...
Text Solution
|
- Which of the following fcc structure contain cations in alternate tetr...
Text Solution
|
- The edge length of unit cell of a metal having molecular weight 75 g m...
Text Solution
|
- An element crystallizes in fcc lattice having edge length 400 pm Calcu...
Text Solution
|
- Match the crystal system/unit cells mentioned in Column I with their c...
Text Solution
|
- In hexagonal system of crystals, a frequently encountered arrangement ...
Text Solution
|
- In hexagonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- In hexagonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- The corrent statement regarding defects is solids in solids is
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- If the unit cell of a mineral has cubic close packed (ccp) array of ox...
Text Solution
|
- Which of the following statements is(are) correct?
Text Solution
|
- The CORRECT statements (s) for cubic close packed (ccp) three dimensio...
Text Solution
|
- A crystalline solid of a pure substance has a face-centred cubic struc...
Text Solution
|
- Consider an ionic solid MX with NaCl structure. Construct a new struct...
Text Solution
|