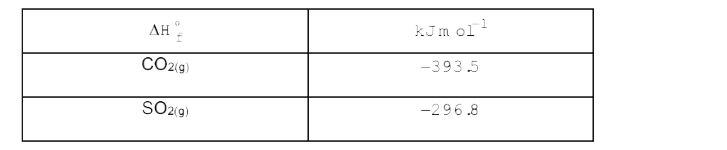
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE MAIN (ARCHIVE)|20 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|44 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMOCHEMISTRY-LEVEL-2
- From given following equations and DeltaH^(@) values, determine the en...
Text Solution
|
- NH(3)(g) + 3Cl(2)(g) rarr NCl(3)(g) + 3HCl(g), " "DeltaH(1) N(...
Text Solution
|
- The combustion of 0.2 mol of liquid carbon disulphide CS2 to give CO2(...
Text Solution
|
- Estimate the average S -F bond energy in SF(6). The values of standard...
Text Solution
|
- Heat of atomisation of NH(3) and N(2)H(4) are "x kcal mol"^(-1) and "...
Text Solution
|
- 2Fe+(3)/(2)O(2)rarr Fe(2)O(3)" "xkJ//mol e 2Fe+O(2) rarr 2FeO" ...
Text Solution
|
- The heat of reaction for N(2)+3H(2) rarr 2NH(3) at 27^(@)C is -91.94kJ...
Text Solution
|
- The heat evolved in the combustion of benzene is given by C(6)H(6) (...
Text Solution
|
- If x(1) , x(2) and x(3) are enthalpies of H-H , O=O and O-H bonds resp...
Text Solution
|
- Calculate the resonance enegry of N(2)O form the following data Delt...
Text Solution
|
- When a certain amount of ethylene was combusted, 6226 kJ heat was evol...
Text Solution
|
- For which one of the following equations DeltaH(r)^(@) equal to DeltaH...
Text Solution
|
- The enthalpy of hydrogenation for 1-pentene is +126 kJ/mol. The enthal...
Text Solution
|
- 1.0litre sample of a mixture of CH(4) and O(2) measured at 25^(@)C and...
Text Solution
|
- The commercial production of water gas utilizes the reaction under sta...
Text Solution
|
- Calculate the heat of neutralisation from the following data: 200mL ...
Text Solution
|
- A cylinder of gas is assumed to contain 11.6 kg of butane (C4H10). If ...
Text Solution
|
- The heat of combusion of glycogen is about 476 kJ mol^(-1) of carbon. ...
Text Solution
|
- Given the equilibrium system NH(4)Cl(s)hArr NH(4)^(+)(aq)+Cl^(-)(aq)...
Text Solution
|
- Difference between the heats of reaction at constant pressure and a co...
Text Solution
|