Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-JEE ADVANCED (ARCHIVE)
- When 1-pentyne (A) is treated with 4N alcoholic KOH at 175^(@)C, it is...
Text Solution
|
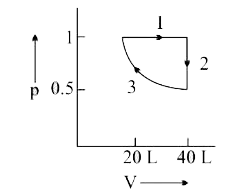
- One mole of a non-ideal gas undergoes a change of state (2.0 atm, 3.0 ...
Text Solution
|
- Two moles of a perfect gas undergo the following processes: a. A rev...
Text Solution
|
- The molar heat capacity , C(v) of helium gas is 3//2R and is independe...
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|
- An insulated vessel contains 1mole of a liquid, molar volume 100mL at ...
Text Solution
|
- When 1mol of a monoatomic ideal gas at TK undergoes adiabatic change u...
Text Solution
|
- A monatomic ideal gas undergoes a process in which the ratio of P to V...
Text Solution
|
- The direct conversion of A to B is difficult hence it is carried out b...
Text Solution
|
- N(2)+3H(2) hArr 2NH(3) Which of the following statements is correct ...
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- For the process H(2)O(l) (1 "bar", 373 K) rarr H(2)O(g) (1"bar", 373 K...
Text Solution
|
- Assertion (A): For every chemical reaction at equilibrium, standard Gi...
Text Solution
|
- Assertion (A) : There is a natural asymmetry between converting work t...
Text Solution
|
- Among the following the state function(s) is (are):
Text Solution
|
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- To an evacuated vessel with movable piston under external pressure of ...
Text Solution
|
- Match the transformation in Column I with appropriate option in Column...
Text Solution
|
- Match the thermodynamic processes given under column I with the expres...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|