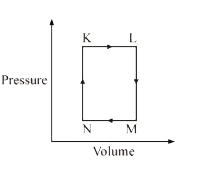
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-JEE ADVANCED (ARCHIVE)
- Among the following the state function(s) is (are):
Text Solution
|
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- To an evacuated vessel with movable piston under external pressure of ...
Text Solution
|
- Match the transformation in Column I with appropriate option in Column...
Text Solution
|
- Match the thermodynamic processes given under column I with the expres...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- A fixed mass ‘m’ of a gas is subjected to transformation of states fro...
Text Solution
|
- The pair of isochoric among the transformation of state is :
Text Solution
|
- An ideal gas in a thermally insulated vessel at internal pressure =P(1...
Text Solution
|
- For the process, H(2)O(l) to H(2)O(g)
Text Solution
|
- Thermal decomposition of gaseous X(2) to gaseous X at 298K takes place...
Text Solution
|
- Thermal decomposition of gaseous X(2) to gaseous X at 298 K takes plac...
Text Solution
|
- The standard state Gibbs free energies of formation of ) C(graphite an...
Text Solution
|
- For a reaction taking place in a container in equilibrium with its sur...
Text Solution
|
- A thermo-dynamical system is changed from state (P(1),V(1)) to (P(2),...
Text Solution
|
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- An ideal gas undergoes a cyclic process as shown in figure .
Text Solution
|
- A reversible cyclic process for an ideal gas is shown below. Here, P, ...
Text Solution
|
- For a reaction A to P, the plots of [A] and [P] with time at temperatu...
Text Solution
|