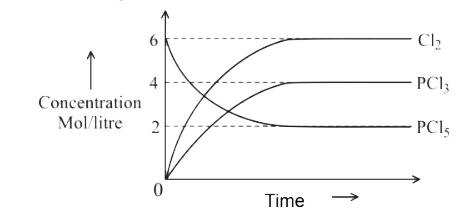
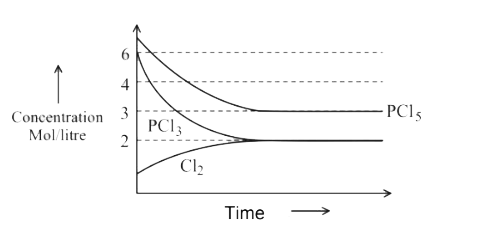
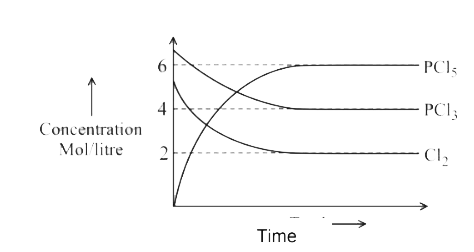
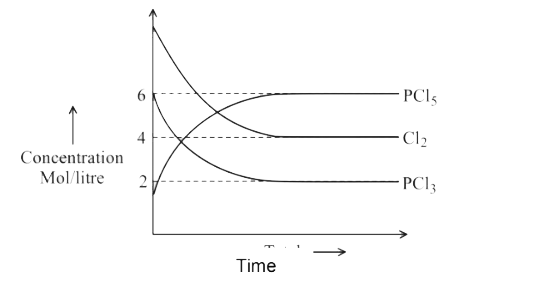
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|24 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|28 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 1|75 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 2
- I(2)(aq)+I^(-)(aq)hArr(aq). We started with 1 mole of I(2) and 0.5 mol...
Text Solution
|
- A gas X(g) is when dissolved in water heat is evolved. Then solubility...
Text Solution
|
- For the reaction PCl(5)(g)hArrPCl(3)(g)+Cl(2)(g) Which of the foll...
Text Solution
|
- The equilibrium constant K(p(1)) and K(p(2)) for the reactions XhArr2Y...
Text Solution
|
- The equilibrium constant K(c) for the reaction P(4)(g)hArr 2P(2)(g) ...
Text Solution
|
- Formaldehyde polymerizes to form glucose according to the reaction, 6H...
Text Solution
|
- The vapour density of N(2)O(4) at a certain temperature is 30. Calcula...
Text Solution
|
- In the reaction equilibrium N(2)O(4) hArr 2NO(2)(g) When 5 mol of ...
Text Solution
|
- At a particular temperature, PCl(5)(g) undergoes 50% dissociation. Th...
Text Solution
|
- Calculate the partial pressure of carbon monoxide from the following d...
Text Solution
|
- The following equilibrium in a saturated solution of NH4Cl. NH(4)Cl(...
Text Solution
|
- The exothermic formation of ClF(3) is represented by thr equation: C...
Text Solution
|
- For the gas phase reaction C(2)H(4)+H(2) hArr C(2)H(6)(DeltaH=-32.7 ...
Text Solution
|
- Given the reaction 2X(g) +Y(g) hArr 7(g) +80 kcal Which combinati...
Text Solution
|
- When pressure is applied to the equilibrium system, IcehArrWater. Whic...
Text Solution
|
- 3 moles of A and 4 moles of B are mixed together and allowed to come i...
Text Solution
|
- In which of the folowing reactions, the system will shift towards the ...
Text Solution
|
- Which of the following factors will increase the solubility of NH(3) g...
Text Solution
|
- An aqueous solution of hydrogen sulphide shows the equilibrium: H(2) S...
Text Solution
|
- The degree of dissociation of I(2) molecule of 1000^(@)C and underatm...
Text Solution
|