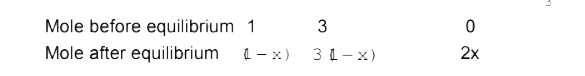Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|24 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|28 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 1|75 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 2
- An aqueous solution of hydrogen sulphide shows the equilibrium: H(2) S...
Text Solution
|
- The degree of dissociation of I(2) molecule of 1000^(@)C and underatm...
Text Solution
|
- For the following reaction through stages I, II and III A overset(I)...
Text Solution
|
- Au(s) hArr Au (l) above mentioned equilibrium is fovoured at
Text Solution
|
- Match the following : Condition for the reaction to be favoured in for...
Text Solution
|
- A sample of air consisting of N(2) and O(2) was heated to 2500 K until...
Text Solution
|
- At 450^(@)C the equilibrium constant K(p) for the reaction N(2)+3H(2) ...
Text Solution
|
- In the dissociation of HI, 20% of HI is dissociated at equilibrium. Ca...
Text Solution
|
- An equilibrium mixture at 300 K contains N(2)O(4) and NO(2) at 0.28 an...
Text Solution
|
- The degree of dissociation is 0.4 at 400 K and 1.0 atm for the gaseous...
Text Solution
|
- For the reaction at 300 K , A(g) rarr B(g) + E(g), DeltaH^(@) = -30 KJ...
Text Solution
|
- For the reaction: 2A(g) + nB(g) rarr 3C(g) If K(p) "and" K(c) are 0....
Text Solution
|
- For the reaction AB(2)(g) rarr AB(g) + B(g) , the initial pressure of...
Text Solution
|
- Two solid comp0ounds X and Y dissociates at a ceritain temperature as ...
Text Solution
|
- How many mole of glycerine should be added to 1 litre of 1 M H(3)BO(3)...
Text Solution
|
- For the reaction H(2)(g) + I(2)(g)hArr2HI(g) at 721 K the value of equ...
Text Solution
|
- In a gaseous reaction A+2B iff 2C+D the initial concentration of B was...
Text Solution
|
- The partial pressure of CH(3)OH((g)), CO((g)) and H(2(g)) in equilibri...
Text Solution
|
- A definite amount of solid NH(4)HS is placed in a flask aleady contain...
Text Solution
|
- The approach to the following equilibrium was observed kinetically for...
Text Solution
|