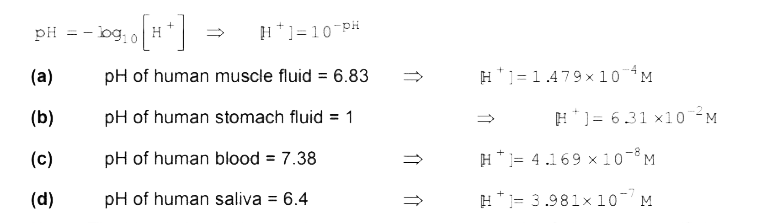Text Solution
Verified by Experts
Topper's Solved these Questions
IONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise LEVEL 1|75 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise LEVEL 2|50 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED ARCHIVE|81 VideosJEE MAIN - 5
VMC MODULES ENGLISH|Exercise PART II : CHEMISTRY (SECTION - 2)|5 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-IONIC EQUILIBRIUM-IN - CHAPTER EXERCISE - K
- Calculate the hydrogen ion concentration in the following biologi...
Text Solution
|
- 20 mL of 0.1N HCI is mixed with 20 ml of 0.1N KOH. The pH of the solut...
Text Solution
|
- A solution contains 10mL of 0.1N NaOH and 10mL of 0.05Na(2)SO(4), pH o...
Text Solution
|
- When 10 mL of 0.1 M acetic acid (pK(a) = 5) is titrated against 10 mL ...
Text Solution
|
- The pK(a) of a weak acid is 4.8 . What should be the ratio of [Acid]/[...
Text Solution
|
- A sample of Na(2)CO(3).H(2)O weighing 0.62 g is added to 10 ml of 0.1 ...
Text Solution
|
- The pH of a solution is 5. to this solution acid was added so that its...
Text Solution
|
- How many millilitree of 6.0 M hydrochloric acid should be used to prep...
Text Solution
|
- A physician wishes to prepare a buffer solution at pH = 3.58 that effi...
Text Solution
|
- The pK(a) of acety1 salicylic acid (aspirin) is 3.5. The pH of gastric...
Text Solution
|
- In a mixture of acetic acid and sodium acetate the ratio of concentrat...
Text Solution
|