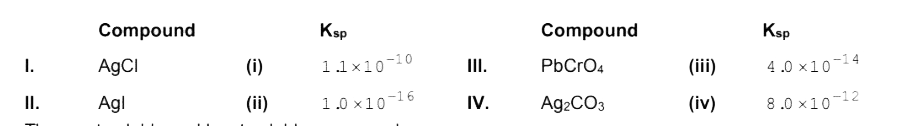A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise LEVEL 2|50 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise LEVEL 2 NUMERICAL VALUE TYPE|15 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - K|10 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED ARCHIVE|81 VideosJEE MAIN - 5
VMC MODULES ENGLISH|Exercise PART II : CHEMISTRY (SECTION - 2)|5 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-IONIC EQUILIBRIUM-LEVEL 1
- When HCI is passed through a saturated solution of common salt, pure N...
Text Solution
|
- In 1L saturated solution of AgCl[K(SP)(AgCl)= 1.6xx10^(-10)], 0.1 mole...
Text Solution
|
- The solubility product (K(sp)) of the following compound are given at ...
Text Solution
|
- A solution contining 0.01 M Zn^(2+) and 0.01 M Cu^(2+) is saturated by...
Text Solution
|
- Some chemists at wished to perpare a saturated solution of a silver co...
Text Solution
|
- Solid Ba (NO(3))(2) is gradually dissolved in 1 1.0 xx 10^(-4)M " " Na...
Text Solution
|
- The conjugate base of H(2)PO(4)^(-) is :
Text Solution
|
- Conjugate base of HO(2)^(-) is :
Text Solution
|
- pH scale was introduced by :
Text Solution
|
- What is the pH value of N/10^(7) KOH solution?
Text Solution
|
- The pH of a 10^(-9)M solution of HCl in water is :
Text Solution
|
- one litre of H(2)O contains 10^(-7) moles [H^(+)]. Degrees of ionizati...
Text Solution
|
- The conjugate bas of hydrazoic acid is
Text Solution
|
- NH(4)^(+) ion in an aqueous solution will behave as :
Text Solution
|
- In the dissociation of bicarbonate ion, the conjugate base involved is...
Text Solution
|
- The decreasing order of the basic character of the following is : (I...
Text Solution
|
- Which of the following is not a Bronsted acid
Text Solution
|
- The compound that is not a Lewis acids is
Text Solution
|
- The following equilibrium is established when hydrogen chloride is dis...
Text Solution
|
- In the equation I(2)+I^(-) rarr, I(3)^(-) which is Lewis base
Text Solution
|