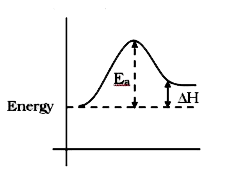
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise Level-2|50 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise Level-2 ( Numerical Value Type for JEE Main )|15 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise Level-0 (Long Answer Type )|6 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL KINETICS -Level-1
- The half life of a first order reaction is 10 minutes. If initial amou...
Text Solution
|
- The rate of a gaseous reaction is given by the expression k[A]^(2)[B]^...
Text Solution
|
- For an endothermic reaction, Delta H represents the enthalpy of the re...
Text Solution
|
- In a first order reaction, 75% of the reactants disappeared in 1.386 h...
Text Solution
|
- For the reaction , N2O4(g)underset(K2)overset(K1)hArr2NO2(g), the rat...
Text Solution
|
- The rate constant, activation energy, and Arrphenius parameter of a ch...
Text Solution
|
- For a first order reaction with rate constant 'k' and initial concentr...
Text Solution
|
- The rate constant is given by the equation k = P.Ze^(-E(a)//RT). Which...
Text Solution
|
- The temperature coefficient for most of the reaction lies between
Text Solution
|
- The raction 2FeCl(3) + SnCl(2) rarr 2FeCl(2) + SnCl(4) is an example ...
Text Solution
|
- The activation energy of a reaction can be determined by
Text Solution
|
- Units of rate constant depend upon
Text Solution
|
- IF the t(1//2) for a first order reaction 0.4 min, the time of or 99.9...
Text Solution
|
- Which of the following is pseudo first order reaction? I. CH(3)COOC(...
Text Solution
|
- For the reaction A + B rarr C + D, doubling the concentration of both ...
Text Solution
|
- In Arrhenius plot, intercept is equal to
Text Solution
|
- When a biochemical reaction is carried out in laboratory from outside ...
Text Solution
|
- A sample of radioactive substance loses half of its activity in 4 days...
Text Solution
|
- A chemical reaction was carried out at 300 K and 280 K. The rate const...
Text Solution
|
- Collision theory is applicable to
Text Solution
|