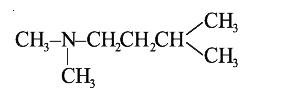A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
VMC MODULES ENGLISH|Exercise ILLUSTRATION|25 VideosAMINES
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|21 VideosAMINES
VMC MODULES ENGLISH|Exercise EFFICIENT|49 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-AMINES-IMPECCABLE
- which of the indicated 'H' is most acidic ?
Text Solution
|
- Correct order of acidic strength in following acids will be:
Text Solution
|
- The magor product formed in the elimination reaction of is:
Text Solution
|
- When CH(3)CH(2) CHCl(2) is treated with "NaNH"(2) , the product formed...
Text Solution
|
- Which of the following is not the metamer of CH3CH2-underset(CH3)under...
Text Solution
|
- Which of the following compound is most acidic?
Text Solution
|
- Which one of the following amines is the weakest base?
Text Solution
|
- Among the following the strongest base is
Text Solution
|
- Which of the following compounds is most basics
Text Solution
|
- Describe the following giving the relevant chemical equation in each ...
Text Solution
|
- In the Sandmeyer's reaction, -NH-X group of diazonium salt is replaced...
Text Solution
|
- Reaction of benzenediazonium chloride with alkaline b - napthol gives ...
Text Solution
|
- The minimum value of -logK(b) will be for the compound
Text Solution
|
- The reagents that can be used to convert benzenediazonium chloride to ...
Text Solution
|
- Acetanilide when treated with bromine in acetic acid gives:
Text Solution
|
- Which reagent is used to get iodo benzene from benzene diazonium acid ...
Text Solution
|
- The correct order of base strength of substituted aniline is
Text Solution
|
- Choose the false statement
Text Solution
|
- Of the following statements: (a) C(6)H(5)N=CH-C(6)H(5) is a Schiff's...
Text Solution
|
- I overset(COCl2) larr "Aniline"overset(C2 H5 MgI)to II. Products I an...
Text Solution
|