Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
ZEN PUBLICATION|Exercise ZEN ADDITIONAL QUESTIONS SECTION (LONG ANSWER [LA] TYPE 2 QUESTIONS)|14 VideosMETALS AND NON METALS
ZEN PUBLICATION|Exercise ZEN ADDITIONAL QUESTIONS SECTION (SHORT ANSWER [SA] TYPE 1 QUESTIONS)|28 VideosCHEMICAL REACTIONS AND EQUATIONS
ZEN PUBLICATION|Exercise ZEN ADDITIONAL QUESTIONS SECTION (LONG - ANSWER [LA]TYPE QUESTIONS )|27 VideosPERIODIC CLASSIFICATION OF ELEMENTS
ZEN PUBLICATION|Exercise QUESTIONS|2 Videos
Similar Questions
Explore conceptually related problems
ZEN PUBLICATION-METALS AND NON METALS-ZEN ADDITIONAL QUESTIONS SECTION (SHORT ANSWER TYPE 2 QUESTIONS)
- Study the following reactions and explain the reactivity. Arrange Cu, ...
Text Solution
|
- Give the most suitable word for the following statements? (i) Metal ...
Text Solution
|
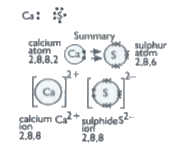
- a. Write a electron dot structure for calcium and sulphur. b. Show t...
Text Solution
|
- A non metal A the largest constituent of air, when heated with hydroge...
Text Solution
|
- a. Write the balanced chemical equations for the extraction of copper ...
Text Solution
|
- Give the steps in the extraction of metals of low and medium reactivit...
Text Solution
|
- A metal 'M' is found in nature as its carbonate. It is used in the gal...
Text Solution
|
- A metal X is used extensively in making aeroplane bodies as it is ligh...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- Match the method of extraction with the metals.
Text Solution
|
- How is impure copper purified by electrolytic refining? Draw a neat la...
Text Solution
|
- Tabulate the changes and compounds formed when Ag, Cu, and Fe articles...
Text Solution
|
- Define the term alloy. Write two advantages of making alloys.
Text Solution
|
- A metal is slowly eaten away in the presence of air and water. To avoi...
Text Solution
|
- Two elements X and Y have atomic number 3 and 5 respectively, give the...
Text Solution
|
- Explain the reactions of different metals with lot water, cold water a...
Text Solution
|
- State the property utilized in the following : (i) Graphite is makin...
Text Solution
|
- (i) Write any two properties of ionic compound. (ii) Show the format...
Text Solution
|
- P,Q and R are 3 elements which undergo chemical reactions according to...
Text Solution
|
- Account for the following: (i) Electrical wire are coated with plast...
Text Solution
|