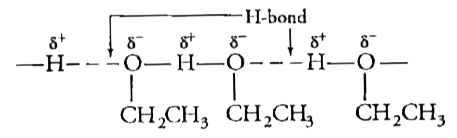
Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (3 MARK)|11 VideosETHERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (7 MARK)|5 VideosETHERS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (7 MARK)|3 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( Long Answer Type Questions ) |11 VideosEXAMINATION PAPER 2018
ARIHANT PUBLICATION|Exercise GROUP C|7 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ETHERS -QUESTIONS FOR PRACTICE (2 MARK)
- Draw the structures of the compounds whose IUPAC names are as follows ...
Text Solution
|
- Draw the structures of the compounds whose IUPAC names are as follows ...
Text Solution
|
- Explain the following with an example. Williamson's ether synthesis
Text Solution
|
- Explain the following with an example. Unsymmetrical ether.
Text Solution
|
- Give the machanism of preparation of ethoxy ethane from ethanol.
Text Solution
|
- Write the mechanism of the following reaction : 2CH(3)CH(2)OHoverset...
Text Solution
|
- Write the names of reagents and equations for the preparation of the f...
Text Solution
|
- Write the names of reagents and equations for the preparation of the f...
Text Solution
|
- Write the names of reagents and equations for the preparation of the f...
Text Solution
|
- Write the names of reagents and equations for the preparation of the f...
Text Solution
|
- Write the reaction of Williamson's synthesis of 2-ethoxy-3-methyl pent...
Text Solution
|
- Give the reason of the higher boiling point of ethanol in comparison t...
Text Solution
|
- Explain why alcohols and ethers of comparable molecular mass have diff...
Text Solution
|
- Explain why O=C=O is non - polar while R-O-R is polar ?
Text Solution
|
- Give reason : (CH(3))(3)C-O-CH(3) on reaction with HI gives (CH(3))(3)...
Text Solution
|
- State the products of the following reactions : CH(3)CH(2)CH(2)OCH...
Text Solution
|
- Predict the products of the following reactions :
Text Solution
|
- Predict the products of the following reactions :
Text Solution
|
- Predict the products of the following reactions : (CH(3))(3)C-OC(2)H...
Text Solution
|