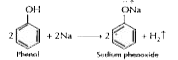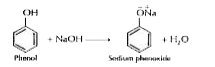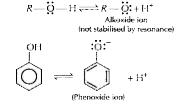Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-SAMPLE PAPER 1 -GROUP B
- Write the IUPAC name of the following complex. [Co(NH(3))(5)Cl]^(2+)
Text Solution
|
- Write the formula for the following complex. Potassium tetrachloridoni...
Text Solution
|
- Name the common elements present as anode mud in the electrolytic refi...
Text Solution
|
- The vapour pressure of water is 12.3 kPa at 300 K. Calculate the vapou...
Text Solution
|
- Explain why NH(3), is basic, while BiH(3) , is only feebly basic?
Text Solution
|
- Which compound in each of the following pairs will react faster in S(N...
Text Solution
|
- How are synthetic detergents better than soaps?
Text Solution
|
- For the reaction, 2A +B to A(2) B, The rate =k[A][B]^(2) with k= 2....
Text Solution
|
- Differentiate between rubbers and plastics on the basis of intermolecu...
Text Solution
|
- Examine the given defective crystal : {:(X^(+),Y^(-),X^(+),Y^(-),X^...
Text Solution
|
- Examine the given defective crystal : {:(X^(+),Y^(-),X^(+),Y^(-),X^...
Text Solution
|
- Examine the given defective crystal : {:(X^(+),Y^(-),X^(+),Y^(-),X^...
Text Solution
|
- How would you account for the following? (a) Transition metals exhi...
Text Solution
|
- Amino acids may be acidic, alkaline or neutral. How does this happen? ...
Text Solution
|
- What is the difference between a colloidal solution and emulsion? What...
Text Solution
|
- A 5% solution (by mass) of cane sugar in water has freezing point of 2...
Text Solution
|
- Discuss briefly giving an example in each case, the role of coordianti...
Text Solution
|
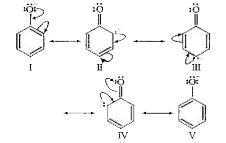
- Give one reaction to show that phenol is acidic in nature.
Text Solution
|
- Describe a method for the identification of primary, secondary and ter...
Text Solution
|
- How would you differentiate between S(N)1 and S(N)2 mechanism of subst...
Text Solution
|