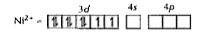Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-SAMPLE PAPER 1 -GROUP C
- A strip of nickel metal is dipped in a 1 molar solution of Ni (NO(3))(...
Text Solution
|
- A strip of nickel metal is dipped in a 1 molar solution of Ni (NO(3))(...
Text Solution
|
- What is the crystal field stabilisation energy? How does the magnitude...
Text Solution
|
- A solution [Ni(H2O)6]^(2+) is green while a solution of [Ni(CN)4]^(2-)...
Text Solution
|
- Distinguish between order and molecularity.
Text Solution
|
- Define rate constant of a reaction .
Text Solution
|
- A first order reaction takes 20 minutes for 25% decomposition. Calcula...
Text Solution
|
- State the products of the following reactions : CH(3)CH(2)CH(2)OCH...
Text Solution
|
- State the products of the following reactions :
Text Solution
|
- State the products of the following reactions : (CH(3))(3)C - OC(2)...
Text Solution
|
- Give the structures and IUPAC names of monohydric phenols of molecula...
Text Solution
|
- Show how are the following alcohols prepared by the reaction of a suit...
Text Solution
|
- An organic compound A (molecular formula (C(8)H(16)O(2)) was hydrolyse...
Text Solution
|
- Explain following Friedel-Crafts acetylation of anisole.
Text Solution
|