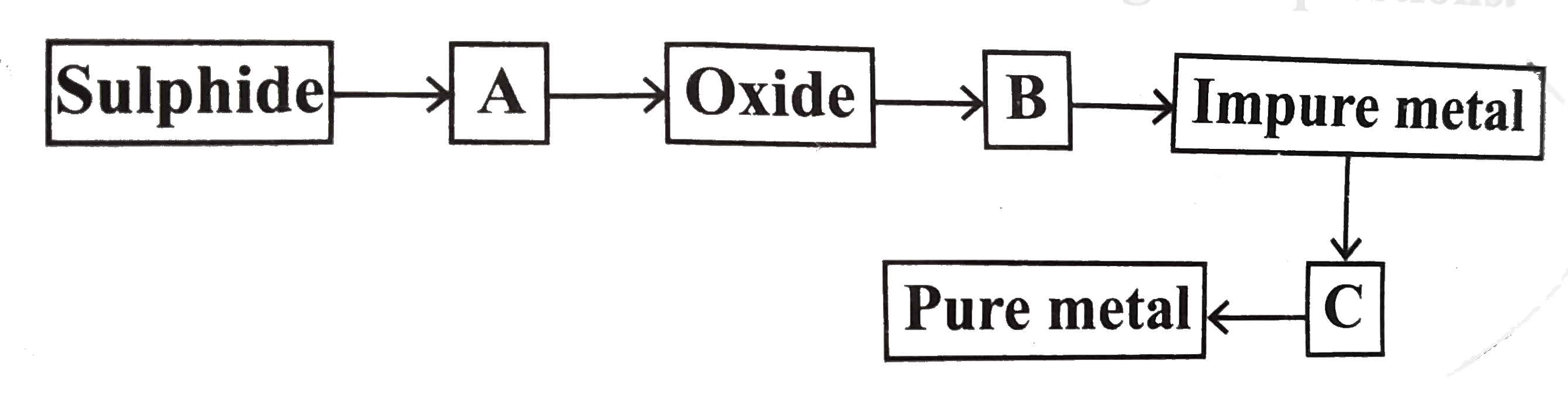
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-METALLURGY-EXERCISE-02
- Neutral refractory material used in furnaces is :
Text Solution
|
- Carbon cannot be used in the reduction of Al(2)O(3) because :
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- Of the following reduction processes, correct processes are :
Text Solution
|
- Consider the following steps: Cu(2)S overset("roast in air")rarr A o...
Text Solution
|
- Main source of lead is galena (PbS). It is converted to Pb by : S...
Text Solution
|
- Ag(2)S + NaCN rarr (A),(A) + Zn rarr (B) (B) is a metal. Hence, (A) ...
Text Solution
|
- The elemental phosphorus is made from rock phosphate, Ca(3)(PO(4))(2) ...
Text Solution
|
- A: Bauxite is purified by leaching process R: Aluminium oxide reacts ...
Text Solution
|
- The process which do use catalysts are
Text Solution
|
- Metallury involves steps :
Text Solution
|
- Which of the following metals is obtained by electrolytic reduction pr...
Text Solution
|
- Which of the following ore is/are oxide ore (s) ?
Text Solution
|
- Which of the following are correctly matched?
Text Solution
|
- In which of the following pair(s) the minerals are converted in to m...
Text Solution
|
- Tin stone (cassiterite) is purified by magnetic seperation method .Na...
Text Solution
|
- The reaction (s) which does (do) not occur in the reduction zone in ...
Text Solution
|
- Which of the following statements(s) is (are) true?
Text Solution
|
- The major role of flourspar (CaF(2)), which is added in small quantiti...
Text Solution
|
- Which of the followgin are correctly matched?
Text Solution
|