A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-GEOMETRICAL OPTICS-EXERCISE -02
- Three containers C(1),C(2) and C(3) have water at different temperat...
Text Solution
|
- Two identical beakers with negligible thermal expansion are filled wit...
Text Solution
|
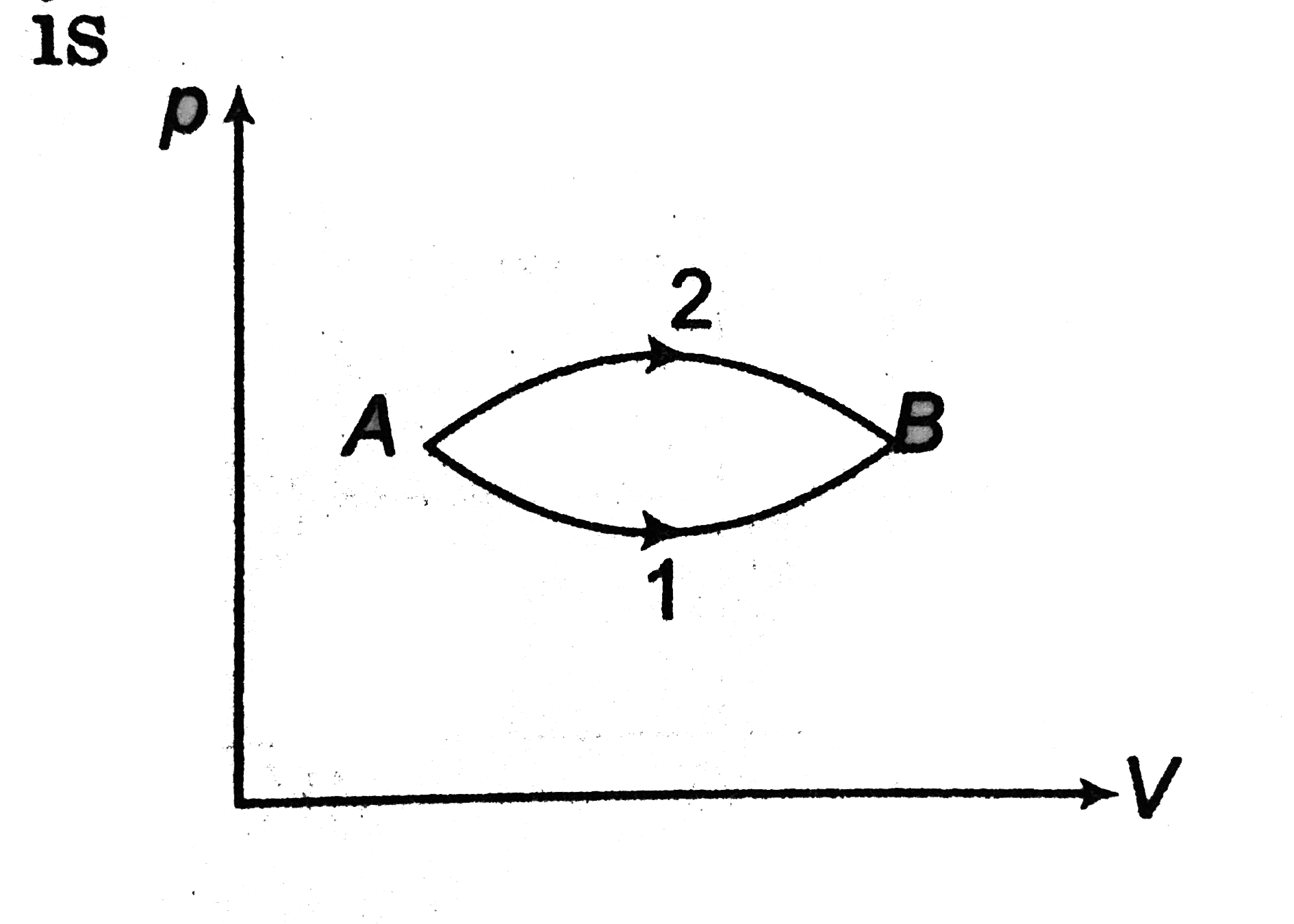
- The figure shows two paths for the change of state of a gas from A to ...
Text Solution
|
- During the melting of a slab of ice at 273 K a atmospheric pressure
Text Solution
|
- T wo substances A and B of equal mass m are heated at uniform rate of ...
Text Solution
|
- A well insulated substance in solid state is heated at a constant rate...
Text Solution
|
- Three closed vessels A,B, and C are at the same temperature T and cont...
Text Solution
|
- A partition divides a container having insulated walls into two compar...
Text Solution
|
- When a gas is heated in a vessel of constant volume , the pressure inc...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- An ideal gas can be expended from an initial state to a certain volume...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is as shown in figur...
Text Solution
|
- When unit mass of water boils to become steam at 100^(@)C, it absorbs ...
Text Solution
|
- N molecules each of mass m of gas A and 2N molecules each of mass 2m o...
Text Solution
|
- A vessel is partitioned in two equal halves by a fixed diathermic sepa...
Text Solution
|
- A closed vessel contains a mixture of two diatomic gases A and B. Mola...
Text Solution
|
- N (lt 100) molecules of a gas have velocities 1,2,3….N km/s respective...
Text Solution
|
- Let vecV, V("m s") and Vp respectively, denote the mean speed, root ...
Text Solution
|
- The following are the P-V diagrams for cyclic processes for a gas. In ...
Text Solution
|
- The internal energy of a system remains constant when it undergoes (...
Text Solution
|