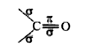
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.2 Preparation of Aldehydes And Ketones )|10 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12. 3 Physical Properties )|60 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12.9 Uses of Carboxylic Acids)
- What type of hybridisation is involved for carbon in a carbonyl group?
Text Solution
|
- Sodium benzoate is used as a food additive. Why ?
Text Solution
|
- Give important industrial uses of formic acid or methanoic acid.
Text Solution
|
- Give important properties of ethanoi acid (acetic acid) also give its ...
Text Solution
|
- Name the constituent of vinegar.
Text Solution
|