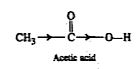
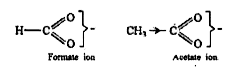Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.9 Uses of Carboxylic Acids)|4 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.7 Physical properties)|4 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12.8 Chemical Reaction)
- Arrange the following in the increasing order of acidity : CHCl2 COOH,...
Text Solution
|
- Formic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Which acid would you expect to be stronger ? CH(3)COOH or HCOOH
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Ethanoic acid is weaker acid than benzoic acid.Why ?
Text Solution
|
- p-Nitrobenzoic acid is a stronger acid than benzoic acid. Justify.
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- Why acetic-acid is a stronger acid than phenol ?
Text Solution
|
- Which acid would you expect to be stronger:
Text Solution
|
- Which acid would you expect to be stronger ?
Text Solution
|
- Why chloroacetic acid is stronger acid than acetic acid ?
Text Solution
|
- Fluoroacetic acid is stronger than acetic acid. Explain why ?
Text Solution
|
- Write HVZ reaction.
Text Solution
|
- Write short note on decarboxylation reaction.
Text Solution
|
- Why trichloro acetic acid is stronger than acetic acid ?
Text Solution
|
- Why dichloroacetic acid is stronger than monochloroacetic acid ?
Text Solution
|
- Fluoroacetic acid is stronger than chloroacetic acid. Explain why ?
Text Solution
|
- Convert the following : Acetic acid to acetaldehyde
Text Solution
|
- Convert acetic acid to methylamine.
Text Solution
|
- Write the chemicat equations when acetic acid reacts with SOCl2, PCl3 ...
Text Solution
|