Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.9 Uses of Carboxylic Acids)|4 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.7 Physical properties)|4 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12.8 Chemical Reaction)
- How will you convert Benzoic acid into benzamide ?
Text Solution
|
- Write short note on Kolbe's electrolysis.
Text Solution
|
- Write short note on Hunsdicker reaction.
Text Solution
|
- Write short note on Esterification
Text Solution
|
- Why is the ester distilled as fast as it is formed during the preparat...
Text Solution
|
- Carboxylic acids do not give the characteristic reactions of carbonyl ...
Text Solution
|
- Why carboxylic acids do not give characteristic reactions of -OH group...
Text Solution
|
- Why do carboxylic acids do not form oximes with NH2OH (hydroxyl amine)...
Text Solution
|
- Give simple chemical tests to distinhuish between the following pairs ...
Text Solution
|
- Give simple chemical tests to distinhuish between the following pairs ...
Text Solution
|
- Give simple chemical tests to distinhuish between the following pairs ...
Text Solution
|
- Give simple chemical tests to distinhuish between the following pairs ...
Text Solution
|
- Give simple chemical tests to distinhuish between the following pairs ...
Text Solution
|
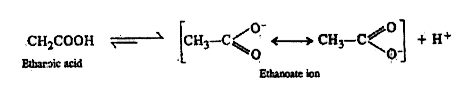
- What makes ethanoic acid a stronger acid than ethanol ?
Text Solution
|
- Complete the following: 2CH3COOH+ Na2CO3 rarr
Text Solution
|
- Complete the following: 2CH3COOH+2Na rarr
Text Solution
|
- Complete the following Reactions. CH(3)COOH + NaOH rarr
Text Solution
|
- Complete the following Reactions. CH(3)COOH + NH(3) rarr
Text Solution
|
- Complete the following reaction:-
Text Solution
|
- Complete the following reaction:-
Text Solution
|