Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Exercise (Multiple Choice Questions ) Level-1 |30 VideosMETALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Exercise (Multiple Choice Questions ) Level-2 |20 VideosMETALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Solved Examples |15 VideosFOOTSTEPS TOWARDS CBSE BOARD
MTG IIT JEE FOUNDATION|Exercise SECTION - C|39 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-METALS AND NON METALS -NCERT SECTION
- What chemical process is used for obtaining a metal from its oxide?
Text Solution
|
- Metallic oxides of zinc, magnesium and copper were heated with the fol...
Text Solution
|
- Which metals do not corrode easily?
Text Solution
|
- What are alloys?
Text Solution
|
- Which of the following pairs will give displacement reactions?
Text Solution
|
- Which of the following methods is suitable for preventing an iron fryi...
Text Solution
|
- An element reacts with oxygen to give a compound with a high melting p...
Text Solution
|
- Food cans are coated with tin and not with zinc because
Text Solution
|
- You are given a hammer, a battery, a bulb, wires and a switch. (a) H...
Text Solution
|
- What are amphoteric oxides? Give two examples of amphoteric oxides.
Text Solution
|
- Name two metals which will displace hydrogen from dilute acids, and tw...
Text Solution
|
- In the electrolytic refining of a metal M, what would you take as the ...
Text Solution
|
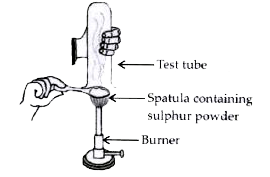
- Pratyush took sulphur powder on a spatula and heated it. He collected ...
Text Solution
|
- State two ways to prevent the rusting of iron.
Text Solution
|
- What type of oxides are formed when non-metals combine with oxygen?
Text Solution
|
- Give reasons (a) Platinum, gold and silver are used to make jeweller...
Text Solution
|
- You must have seen tarnished copper vessels being cleaned with lemon o...
Text Solution
|
- Differentiate between metal and non-metal on the basis of their chemic...
Text Solution
|
- A man went door to door posing as a goldsmith. He promised to bring ba...
Text Solution
|
- Give reasons why copper is used to make hot water tanks and not steel ...
Text Solution
|