Rajesh and Mahesh have defective haemoglobin due to genetic disorders. Rajesh has too few globin molecules while Mahesh has incorrectly functioning globin molecules.Identify the disorder they are suffering from.
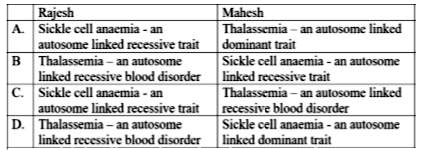
Rajesh and Mahesh have defective haemoglobin due to genetic disorders. Rajesh has too few globin molecules while Mahesh has incorrectly functioning globin molecules.Identify the disorder they are suffering from.


Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
Sickle cell anemia is a genetic disorder where the body produces an abnormal hemoglobin called hemoglobin S. Red blood cells are normally flexible and round, but when the hemoglobin is defective, blood cells take on a “sickle” or crescent shape. Sickle cell anemia is caused by mutations in a gene called HBB. It is an inherited blood disorder that occurs if both the maternal and paternal copies of the HBB gene are defective. In other words, if an individual receives just one copy of the defective HBB gene, either from mother or father, then the individual has no sickle cell anemia but has what is called “sickle cell trait”. People with sickle cell trait usually do not have any symptoms or problems but they can pass the mutated gene onto their children. There are three inheritance scenarios that can lead to a child having sickle cell anemia: - Both parents have sickle cell trait - One parent has sickle cell anemia and the other has sickle cell trait - Both parents have sickle cell anemia If one parent has sickle cell anemia and the other has sickle cell trait, there is __________that their children will have sickle cell anemia and ___________will have sickle cell trait.
Sickle cell anemia is a genetic disorder where the body produces an abnormal hemoglobin called hemoglobin S. Red blood cells are normally flexible and round, but when the hemoglobin is defective, blood cells take on a “sickle” or crescent shape. Sickle cell anemia is caused by mutations in a gene called HBB. It is an inherited blood disorder that occurs if both the maternal and paternal copies of the HBB gene are defective. In other words, if an individual receives just one copy of the defective HBB gene, either from mother or father, then the individual has no sickle cell anemia but has what is called “sickle cell trait”. People with sickle cell trait usually do not have any symptoms or problems but they can pass the mutated gene onto their children. There are three inheritance scenarios that can lead to a child having sickle cell anemia: - Both parents have sickle cell trait - One parent has sickle cell anemia and the other has sickle cell trait - Both parents have sickle cell anemia If both parents have sickle cell trait, then there is _______________of the child having sickle cell anemia.
Sickle cell anemia is a genetic disorder where the body produces an abnormal hemoglobin called hemoglobin S. Red blood cells are normally flexible and round, but when the hemoglobin is defective, blood cells take on a “sickle” or crescent shape. Sickle cell anemia is caused by mutations in a gene called HBB. It is an inherited blood disorder that occurs if both the maternal and paternal copies of the HBB gene are defective. In other words, if an individual receives just one copy of the defective HBB gene, either from mother or father, then the individual has no sickle cell anemia but has what is called “sickle cell trait”. People with sickle cell trait usually do not have any symptoms or problems but they can pass the mutated gene onto their children. There are three inheritance scenarios that can lead to a child having sickle cell anemia: - Both parents have sickle cell trait - One parent has sickle cell anemia and the other has sickle cell trait - Both parents have sickle cell anemia If both parents have sickle cell trait, then there is _______________of the child having sickle cell trait.
Sickle cell anemia is a genetic disorder where the body produces an abnormal hemoglobin called hemoglobin S. Red blood cells are normally flexible and round, but when the hemoglobin is defective, blood cells take on a “sickle” or crescent shape. Sickle cell anemia is caused by mutations in a gene called HBB. It is an inherited blood disorder that occurs if both the maternal and paternal copies of the HBB gene are defective. In other words, if an individual receives just one copy of the defective HBB gene, either from mother or father, then the individual has no sickle cell anemia but has what is called “sickle cell trait”. People with sickle cell trait usually do not have any symptoms or problems but they can pass the mutated gene onto their children. There are three inheritance scenarios that can lead to a child having sickle cell anemia: - Both parents have sickle cell trait - One parent has sickle cell anemia and the other has sickle cell trait - Both parents have sickle cell anemia Sickle cell anemia is a/ an ______________________ disease.
Sickle cell anemia is a genetic disorder where the body produces an abnormal hemoglobin called hemoglobin S. Red blood cells are normally flexible and round, but when the hemoglobin is defective, blood cells take on a “sickle” or crescent shape. Sickle cell anemia is caused by mutations in a gene called HBB. It is an inherited blood disorder that occurs if both the maternal and paternal copies of the HBB gene are defective. In other words, if an individual receives just one copy of the defective HBB gene, either from mother or father, then the individual has no sickle cell anemia but has what is called “sickle cell trait”. People with sickle cell trait usually do not have any symptoms or problems but they can pass the mutated gene onto their children. There are three inheritance scenarios that can lead to a child having sickle cell anemia: - Both parents have sickle cell trait - One parent has sickle cell anemia and the other has sickle cell trait - Both parents have sickle cell anemia The following statements are drawn as conclusions from the above data (Kenya). I. Patients with SCD (Sickle Cell Disease) are less likely to be infected with malaria. II. Patients with SCD (Sickle Cell Disease) are more likely to be infected with malaria. III. Over the years the percentage of people infected with malaria has been decreasing. IV. Year 2000 saw the largest percentage difference between malaria patients with and without SCD. Choose from below the correct alternative.
Read the passage given below and answer the following questions : The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionic arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as point charges in case of anions or dipoles in case of neutral molecules. The five d orbitals in an isolated gaseous metal atom/ion have same energy, i.e., they are degenerate. This degeneracy is maintained if a spherically symmetrical field of negative charges surrounds the metal atom/ ion. However, when this negative field is due to ligands (either anions or the negative ends of dipolar molecules like NH_(3) and H_(2)O ) in a complex, it becomes asymmetrical and the degeneracy of the d orbitals is lifted. It results in splitting of the d orbitals. A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent ?
In a ideal crystal there nust be regular repeating arrangement of the constuting particles and its entropy must be zero at absolute zero at absolute zero temperature. However, it is impossible to obtain an ideal crystal and it suffers from certain defects called imperfections. In pure crystal these defects arise either due to disorder or dislocation of the movement of the particles even at absolute zero temperature. Such defect increases with rise in temperature. In addition ti this, certain defects arise due to the pressure of some impurities. Such defects not only modify the existing properties of the crystalline solid but also impart certain new characteritics to them. In pure crystal, e.g, silicon or germanium at 0K, the electrons are prsent in fully occupied lowest energy states and are not xpected to conduct any electricity. However at temperature above 0K, some electron leave their bonds and become free to move in the crystal lattice, giving rise to and become free to move in the crystal lattice, giving rise to electrical conductivity. The electron deficient bonds, called holes (+vely charged) and thermally mobile electrons move in opposite direction under the electric field. Stoichiometric ppoint defects include (a) Schottky defects, which arise due to missing of both cations and anions from their lattice sites without disturbing the stoichiometry and (b) Frenked defects, which arise due to misplacement of certian ions in the crystal lattice. The former defect gives rise to no change of density. Another type of defects are non-stoichometry defects where the cetions and anion are not present in the stoichiometry ratio. In metal excess defect, metal ions or positive ions are in excess as compared to anions of non-metals stoichiometrycally. On the other hand in metal deficiency defect, the cations are in lesser proportion than stoichiometric value. Since the crystal is neutral electrically, the balance of charge is maintained by free electrons or extra positive charges. The metal excess defects gives rise to conduction of electricity due to the presence of free electrons. Also crystals having metal excess defects are paramagnetic and coloured due to the presence of electrons in the anion vacancies. Impurity defects arise when some foreign atoms are present at the lattice sites in place of the host atoms or at the vacant interstitial sites. When 15 group elements like P or are doped into Si or Ge, the added impurity atoms occupy the lattice sites forming four covalent bonds with 4 Si/Ge atoms leaving an extra electron free to move. Such a crystal is said to be n-type semi conductor because the conduction of electricity is due to movement of extra unbounded electrons. If doping of a covalent crystal of 14 group elements are caused by addition of small amounts of elements are caused by addition of small amounts of elements of group 13, e.g, Al or Ga with three valence electrons, one covalent bond formed will be electron deficient and acts as a positive hole. The presence of such holes in the crystal leads to electrical conductivity and the the crystal is said to be p-type semiconductor. Lattice defect per 10^(15)NaCl is 1. What is the number of lattice defects in 1 mole of NaCl?
In a ideal crystal there nust be regular repeating arrangement of the constuting particles and its entropy must be zero at absolute zero at absolute zero temperature. However, it is impossible to obtain an ideal crystal and it suffers from certain defects called imperfections. In pure crystal these defects arise either due to disorder or dislocation of the movement of the particles even at absolute zero temperature. Such defect increases with rise in temperature. In addition ti this, certain defects arise due to the pressure of some impurities. Such defects not only modify the existing properties of the crystalline solid but also impart certain new characteritics to them. In pure crystal, e.g, silicon or germanium at 0K, the electrons are prsent in fully occupied lowest energy states and are not xpected to conduct any electricity. However at temperature above 0K, some electron leave their bonds and become free to move in the crystal lattice, giving rise to and become free to move in the crystal lattice, giving rise to electrical conductivity. The electron deficient bonds, called holes (+vely charged) and thermally mobile electrons move in opposite direction under the electric field. Stoichiometric ppoint defects include (a) Schottky defects, which arise due to missing of both cations and anions from their lattice sites without disturbing the stoichiometry and (b) Frenked defects, which arise due to misplacement of certian ions in the crystal lattice. The former defect gives rise to no change of density. Another type of defects are non-stoichometry defects where the cetions and anion are not present in the stoichiometry ratio. In metal excess defect, metal ions or positive ions are in excess as compared to anions of non-metals stoichiometrycally. On the other hand in metal deficiency defect, the cations are in lesser proportion than stoichiometric value. Since the crystal is neutral electrically, the balance of charge is maintained by free electrons or extra positive charges. The metal excess defects gives rise to conduction of electricity due to the presence of free electrons. Also crystals having metal excess defects are paramagnetic and coloured due to the presence of electrons in the anion vacancies. Impurity defects arise when some foreign atoms are present at the lattice sites in place of the host atoms or at the vacant interstitial sites. When 15 group elements like P or are doped into Si or Ge, the added impurity atoms occupy the lattice sites forming four covalent bonds with 4 Si/Ge atoms leaving an extra electron free to move. Such a crystal is said to be n-type semi conductor because the conduction of electricity is due to movement of extra unbounded electrons. If doping of a covalent crystal of 14 group elements are caused by addition of small amounts of elements are caused by addition of small amounts of elements of group 13, e.g, Al or Ga with three valence electrons, one covalent bond formed will be electron deficient and acts as a positive hole. The presence of such holes in the crystal leads to electrical conductivity and the the crystal is said to be p-type semiconductor. The type of semiconduction shown by crystal capable of showing Schottky defect, will be :
In a ideal crystal there nust be regular repeating arrangement of the constuting particles and its entropy must be zero at absolute zero at absolute zero temperature. However, it is impossible to obtain an ideal crystal and it suffers from certain defects called imperfections. In pure crystal these defects arise either due to disorder or dislocation of the movement of the particles even at absolute zero temperature. Such defect increases with rise in temperature. In addition ti this, certain defects arise due to the pressure of some impurities. Such defects not only modify the existing properties of the crystalline solid but also impart certain new characteritics to them. In pure crystal, e.g, silicon or germanium at 0K, the electrons are prsent in fully occupied lowest energy states and are not xpected to conduct any electricity. However at temperature above 0K, some electron leave their bonds and become free to move in the crystal lattice, giving rise to and become free to move in the crystal lattice, giving rise to electrical conductivity. The electron deficient bonds, called holes (+vely charged) and thermally mobile electrons move in opposite direction under the electric field. Stoichiometric ppoint defects include (a) Schottky defects, which arise due to missing of both cations and anions from their lattice sites without disturbing the stoichiometry and (b) Frenked defects, which arise due to misplacement of certian ions in the crystal lattice. The former defect gives rise to no change of density. Another type of defects are non-stoichometry defects where the cetions and anion are not present in the stoichiometry ratio. In metal excess defect, metal ions or positive ions are in excess as compared to anions of non-metals stoichiometrycally. On the other hand in metal deficiency defect, the cations are in lesser proportion than stoichiometric value. Since the crystal is neutral electrically, the balance of charge is maintained by free electrons or extra positive charges. The metal excess defects gives rise to conduction of electricity due to the presence of free electrons. Also crystals having metal excess defects are paramagnetic and coloured due to the presence of electrons in the anion vacancies. Impurity defects arise when some foreign atoms are present at the lattice sites in place of the host atoms or at the vacant interstitial sites. When 15 group elements like P or are doped into Si or Ge, the added impurity atoms occupy the lattice sites forming four covalent bonds with 4 Si/Ge atoms leaving an extra electron free to move. Such a crystal is said to be n-type semi conductor because the conduction of electricity is due to movement of extra unbounded electrons. If doping of a covalent crystal of 14 group elements are caused by addition of small amounts of elements are caused by addition of small amounts of elements of group 13, e.g, Al or Ga with three valence electrons, one covalent bond formed will be electron deficient and acts as a positive hole. The presence of such holes in the crystal leads to electrical conductivity and the the crystal is said to be p-type semiconductor. In the crystal of Fe_(0.93) O, the percentage of Fe(III) will be:
CBSE MODEL PAPER-SAMPLE PAPER 2022-Questions in lieu of diagram based questions for VI candidates (Section - C)
- Rajesh and Mahesh have defective haemoglobin due to genetic disorders....
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- A biology student after studying about the different levels of hormone...
Text Solution
|
- Domestic wheat, which has 42 chromosomes, is probably hexaploid (6n), ...
Text Solution
|
- The following are results of crossing a female fly (AaBb) with a male ...
Text Solution
|
- On the ribosome, mRNA binds and two sites in the for subsequent amin...
Text Solution
|
- The main reason for the presence of both a leading and a lagging stran...
Text Solution
|
- In a cell, DNA transcription is halted when
Text Solution
|